肌腱韧带等各种3D细胞组织材料构建体培养与与机械特性测试双功能系统
型号:
联系人:李先生
联系电话:18618101725
品牌:
肌腱韧带等各种3D细胞组织材料构建体培养与与机械特性测试双功能系统
te色:
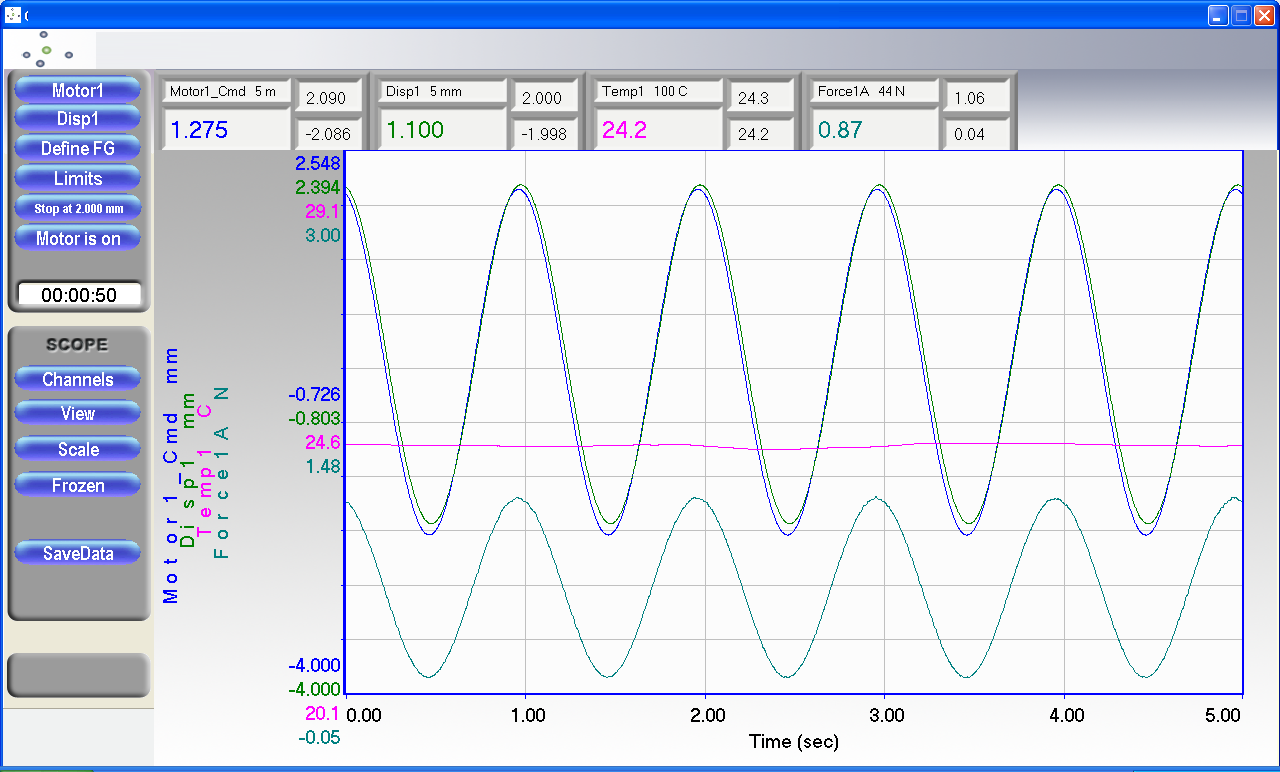
1、刺激培养期间同时进行实时应力应变与位移关系,刚度等自动测量,力、位移、环境温度等
2、具有材料性能测量能力和基于过程调节组织工程医疗产品的生物反应器,测量得到的结果(例如刚度)可作为培养和刺激构建物时变化过程的指标,系统根据测量结果反馈自动修改刺激配置,以减少研究人员的人工干预,自动的将构建物从初的接种状态培养成可移植状态.
3.测试结果一张图实时可视化展示,一键生成报告。
4、可根据培养期间实时测量的反馈,调整力与电、氧气、二氧化碳、氮气、PH值等细胞组织生长的营养培养环境,确保细胞快速生长和繁殖的环境是里想的,实现了从长期培养到表征和评估的wu缝过渡,是组织工程与再生医学创新研究强有力的生物反应器系统。
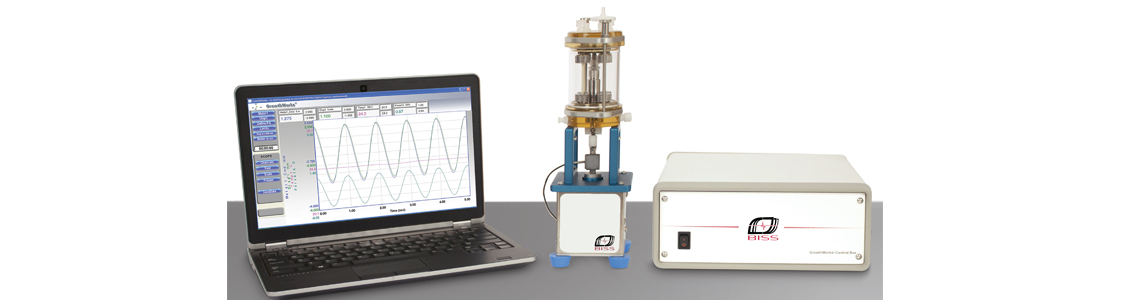
体外培养生物反应器可为肌腱、韧带、水凝胶等构建物提供轴向的压缩/拉伸应力刺激,进行细胞分化、肌腱刺激、药物检测等研究。生物反应器配有多种固定装置,适用于不同的样品,并可设定频率、振幅和载荷等参数来实现不同的实验设计。密封设计te,保证wu菌的同时减少了阻力,使连杆可以自由运动。培养室和夹具都有多种型号,可根据实验需要自行选择.
- 标本长度0-30mm;
- 约23毫升样品室容积;
- 反应室两面均为透明可拆卸窗口,可使用仪器进行光学监测;
- 反应室由生物惰性材料制成,可 121 C,17-20 psi高压湿热灭菌;
- 密封腔室采用te的波纹管设计;
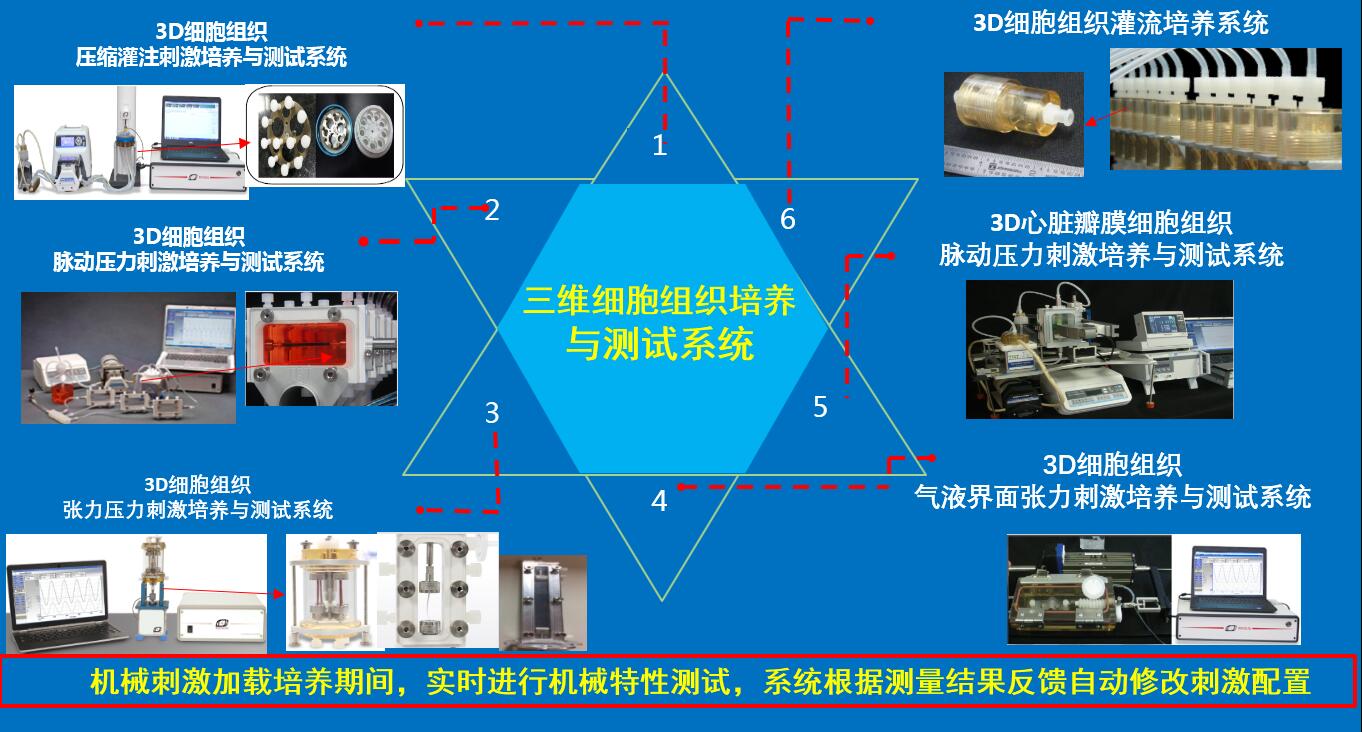
另外提供可以用于包括三维软骨、血管、肌腱韧带、皮肤、心脏瓣膜、骨组织在内的等各种3D细胞组织材料构建体机械力加载培养与机械特性实时测量,可偶联电刺激的各种三维构建体应力刺激培养与机械特性实时测试分析系统。
生物反应器腔由生物惰性,可高压灭菌的材料制成,有助于对具有大纵横比的样品进行振荡压缩/拉伸轴向刺激。腔室可以与各种构造材料一起使用,从脱细胞的肌腱和韧带到聚合的水凝胶。腔室可容纳大30×3 mm的样品。多种抓握方式允许各种具有不同材料特性的结构受到刺激。te的密封为腔室提供机械馈通,同时在wu菌环境中促进轴向运动,并且阻力小。三种不同的腔室设计允许单个样品或多样品刺激。all腔室均可与灌注系统一起使用,以在样品周围提供对流介质传输。
Chamber Options
L30-1X: Single sample per chamber, 30 mm long with 23 mL compartment volume
L30-4C: Either 2 or 4 samples per chamber with 80 mL compartment volume
L150-1C: Single sample per chamber, 150 mm long with 71 mL compartment volume
BiSS bioreactors provide a controllable, 3D environment for stimulating physiological conditions in vitro. The system imparts mechanical tension/compression to a 3D sample. Applications include investigating cell function, modulating the growth and development of engineered tissues, or acting as a test bed for drug and regenerative medicine technologies.
Chambers
Fabricated out of bioinert, autoclavable materials, the bioreactor chamber facilitates oscillatory compressive/tensile axial stimulation to samples with a large aspect ratio. The chambers can be used with a variety of construct materials from decellularized tendons and ligaments to polymerized hydrogels. The chamber accommodates samples up to 30 × 3 mm. Multiple grip styles allow for a wide range of constructs with different material characteristics to be stimulated. A unique seal provides a mechanical feed-through to the chamber, while facilitating axial motion in a sterile environment with minimal resistance. Three different chamber designs allow either single sample or multi-sample stimulation. All chambers can be used with a perfusion system to provide convective media transport around the sample.
Grip Options
? Clamp: mechanical clamp vice grips with screw locking mechanism; allows sample to be installed in grips outside of chamber? In Situ Integrated construct mold and porous Polymerization: grips to enable mechanical stimulation of hydrogel scaffolds
? Custom Grips: custom designed for specific scaffold textures or geometries
GrowthWorks Control System
The controller with integrated motor drives, communicates with the laptop using a network cable. GrowthWorks can be configured to run four stimulators and monitor up to 8 transducers, allowing the researcher to customize the system functionality. The controller can be customized with additional modules for applications requiring automation features or additional axes of mechanical stimulation. Simple and adaptable, the GrowthWorks provides an ideal control platform for mechanically stimulated .Mechanical Stimulator
The bioreactor system includes the tension/compression (T/C) mechanical stimulator. Featuring a 200 N linear motor, the stimulator is lightweight, compact,corrosion resistant, and compatible with most standard incubators. The TC stimulator controls both load and displacement, and can be used with any of the bioreactor chambers.相关文献
PublicationsBISS Bioreactor Systems in Current Literature
Patents
Instrumented bioreactor with material property measurment capability and process-based adjustment for conditioning tissue engineered medical products.US pat no 7410792. August 12, 2008
Bioreactor with plurality of chambers for conditioning intravascular tissue engineered medical products. US pat no 7348175. March 25, 2008
Cell seeding module including an apparatus and method for seeding cells on a sample or specimen. US pat no 8173420. May 8, 2012.
Peer Reviewed Publications
Angelidis IK, Thorfinn J, Connolly ID, Lindsey D, Pham HM, Chang J. Tissue Engineering of Flexor Tendons: The Effect of a Tissue Bioreactor on Adipoderived Stem cell-Seeded and Fibroblast-Seeded Tendon Constructs. J Hand Surg Am. 2010 Sep; 35(9): 1466-72.
Woon Cy, Kraus A, Raghavan SS, Pridgen BC, Megerle K, Pham H, Chang J. Three-Dimensional-Construct Bioreactor Conditioning in Human Tendon Tissue Engineering. Tissue Eng Part A. 2011 July 1: Epublished ahead of print
Tran SC, Cooley AJ, Elder SH. Effects of a Mechanical Stimulation Bioreactor on Tissue Engineered, Scaffold-Free Cartilage. Biotechnology and Bioengineering. 2011; 108:1421-1429.Saber S, Zhang AY, Ki SH, Lindsey DP, Smith RL, Riboh J, Pham H, Chang J. Flexor Tendon Tissue Engineering: Bioreactor Cyclic Strain Increases Construct Strength. Tissue Engineering A. 2010 Jun 16(6): 2085-90.
Fischer LJ, McIlhenny S, Tulenko T, Golesorkhi N, Zhang P, Larson R, Lombardi J, Shapiro I, DiMuzio P. Endothelial Differentiation of Adipose-Derived Stem Cells: Effects of Endothelial Cell Growth Supplement and Shear Force. Journal of Surgical Research. 2009 March; 152 (1):157-166. PubMed PMID 19883577.
Harris LJ, Abdollahi H, Zhang P, McIlhenny S, Tulenko T, DiMuzio PJ. Differentiation of Adult Stem Cells into Smooth Muscle for Vascular Tissue Engineering. Journal of Surgical Research. Article in Press [Epub ahead of print] September 4, 2009. PubMed PMID 19959190.
McIlhenny S, Hager ES, Grabo DJ, DiMatteo C, Shapiro IM, Tulenko T, DiMuzio PJ. Linear Shear Conditioning Improves Vascular Graft Retention of Adipose-Derived Stem Cells by Upregulation of a5?1 Integrin. Tissue Engineering Part A. 2010 Jan; 16(1): 245-255.
Klein TJ, Malda J, Sah RL, Hutmacher DW, Tissue Engineering of Articular Cartilage with Biomimetic Zones. Tissue Engineering Part B. 2009 Feb 9 PubMed PMID 19203206.
Cartmell SH, Porter BD, Garcia AJ, Guldberg RE, Effects of Medium Perfusion Rate on Cell-Seeded Three-Dimensional Bone Constructs In Vitro. Tissue Eng. 2003 Dec;9(6):1197-203.
McClure MJ, Sell SA, Ayres CE, Simpson DG, Bowlin GL. Electrospinning-aligned and random polydioxanon-polycaprolactone-silk-fibroin-blended scaffolds: geometry for a vascular matrix. Biomedical Materials. 2009; 4(5). PubMed PMID 19815970.
Mohan N, Nair PD, Tabata Y. Growth factor-mediated effects on chondrogenic differentiation of mesenchymal stem cells in 3D semi-IPN poly(vinylalcohol)-poly(caprolactone) scaffolds. J Biomed Mater Res A. 2010 Feb 2. [Epub ahead of print] PubMed PMID: 20128001.
Porter BD, Lin AS, Peister A, Hutmacher D, Guldberg RE, Noninvasive image analysis of 3D construct mineralization in a perfusion bioreactor. Biomaterials. 2007 May; 28(15):2525-33. Epub 2007 Jan 26.
Sell SA, McClure MJ, Barnes CP, Knapp DC, Walpoth BH, Simpson DG, Bowlin GL. Electrospun polydioxanone-elastin blends: potential for bioresorbably vascular grafts. Biomedical Materials. 2006; 1(2).PubMed PMID 18460759.
Smith MJ, McClure MJ, Sell SA, Barnes CP, Walpoth BH, Simpson DG, Bowlin GL. Suture-reinforced electrospun polydioxanone-elastin small-diameter tubes for use in vascular tissue engineering: A feasibility study. Acta Biomaterialia. 2008 Jan;4(1):58-66. PMID 17897890.
Voge CM, Kariolis M, MacDonald RA, Stegemann JP. Directional conductivity in SWNT-collagen-fibrin composite biomaterials through strain-induced matrix alignment. J Biomed Mater Res A. 2008 Jul;86(1):269-77. PubMed PMID: 18428799.
Michael J. McClure, Scott A. Sell, David G. Simpson, Beat H. Walpoth, Gary L. Bowlin. A three-layered electrospun matrix to mimic native arterial architecture using polycaprolactone, elastin, and collagen: A preliminary study. Acta Biomaterialia. Vol. 6, Issue 7, July 2010, Pages 2422-2433.
Dr. Jan Hansmann, Florian Groeber, Alexander Kahlig, Claudia Kleinhans, Heike Walles. Bioreactors in tissue engineering--principles, applications and commercial constraints. Biotechnology Journal. Vol. 8, Issue 2, 2013.
Johan Thorfinn, I.K. Angelidis, L. Gigliello, H.M. Pham, D. Lindsey, J. Chang. Bioreactor optimization of tissue engineered rabbit flexor tendons in vivo. The Journal of Hands Surgery. (Eur Vol.) Feb. 2012 vol. 37 no. 2 pages 109-114.
Presentations
Christopher M. Voge, Mihalis Kariolis, Rebecca A. MacDonald, Jan P. Stegemann, Directional Conductivity in Protein-Nanotube Biomaterials through Strain-Induced Matrix Alignment. 8th World Biomaterials Congress. Amsterdam, Netherlands, June 2008.
S Saber. Stanford University Medical Center, Department of Plastic Surgery, Flexor Tendon Tissue Engineering: Cyclic Strain Increases Construct Strength and Tendon Architecture. Plastic Surgery Research Council. Springfield, Illinois, May 2008. Also presented at the California Society of Plastic Surgeons, Dana Point, California, June 2008.
BD Porter, A Peister, D Hutmacher, RE Guldberg, Dynamic Culture Conditions Modulate Mineralization Matrix Deposition, Growth Rate, and Particle Size Within Large 3-D Constructs. Transactions of the 2006 Summer Bioengineering Conference, Amelia Island, Florida, June 2006.
BD Porter, A Peister, D Hutmacher, RE Guldberg, In Vitro Perfusion Accelerates the Rate of Mineralized Matrix Formation Within 3-D Constructs by Increasing both the Number and Size of Mineralization Sites. Transactions of the 52nd Annual Orthopaedic Research Society, Chicago, Illinois, March 2006.
BD Porter, Roger Zauel, D Hutmacher, RE Guldberg, D Fyhrie, Perfusion Significantly Increases Mineral Production Inside 3-D PCL Composite Scaffolds. Regenerate International Conference and Exposition, Atlanta, Georgia, June 2005. Also presented at the American Society for Mechanical Engineering Summer Bioengineering Meeting, Vail, Colorado, June 2005. Also presented at Transactions of the 51st Annual Orthopaedic Research Society Meeting, Washington, D.C., February 2005.
Posters
S.E.McIlhenny, D.J.Grabo, N.A. Tarola, P.Zhang, I.M.Shapiro, T.N.Tulenko, and P.J.DiMuzio, Shear Conditioning of Adipose-Derived Stem Cells Increases Retention on Decellularized Vein Grafts. Biomedical Engineering Society Meeting, Los Angeles, California, September 2007.
Whitlock, Patrick, Knutson, James, Smith, Thomas L., Van Dyke Mark E., Shilt, Jeffrey S., Koman, L. Andrew, Poehling, Gary G., Effects of Mechanical Stimulation on a Cell-Seeded Scaffold Developed for Tendon and Ligament Regeneration. Transactions of the 6th Combined Meeting of the Orthopaedic Research Society, Honolulu, Hawaii, October 2007. Also presented at the Transactions of the 54th Annual Orthopaedic Research Society Meeting, San Francisco, California, March 2008.
Mechanical Stimulation in the Literature
Reviews
Barrilleaux, B., et al. 2006. Tissue Engineering. "Review: of Ex Vivo Engineering of Living Tissues with Adult Stem Cells." Oct 1 (on line publishing).
Bilodeau, K. and Mantovani, D. 2006. Tissue Engineering. "Bioreactors for tissue engineering focus on mechanical constraints, A comparative review." Aug: 12 (8) 2367-83.
Ratner, B., et al. 1996. Biomaterials Science: An Introduction to Materials in Medicine. Academic Press. San Diego, CA.
Wendt, D., et. al. . 2006. Biorheology. "Uniform tissues engineered by seeding and culturing cells in 3D scaffolds under perfusion at defined oxygen tensions." 43 (3-4): 418-488.
Mcllhenny, S., et al. 2009. Tissue Engineering. "Linear Shear Conditioning Inproves Vascular Graft Retention of Adipose-Derived Stem Cells by Upregulation." Sept. 21 (15).
Juliane Rauh, Falk Milan, Klaus-Peter Gunther, and Maik Stiehler. Tissue Engineering. "Bioreactor Systems for Bone Tissue Engineering." August 2011, 17(4): 263-280.
Bone
Braccini, A. et al. 2005. Stem Cells. "Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts." Sep 23 (8): 1066-72.
Shawn Pl Grogan, Sujata Sovani, Chantal Pauli, Jianfen Chen, Andreas Hartmann, Clifford W. Colwell Jr., Marin K. Lotz, and Darryl D. D'Lima. "Effects of Perfusion and Dynamic Loading on Human Neocartilage Formation of Alginate Hydrogels." Tissue Engineering Part A. September 2012, 18(17-18): 1784-1792.
Vascular
Bouhout S, Perron E, Gauvin R, Bernard G, Ouellet G, Cattan V, Bolduc S. "InVitro Reconstruction of an Autologous, Watertight, and Resistant Vesical Equivalent." Tissue Eng Part A. 2010 Feb 11. [Epub ahead of print] PubMed PMID:20014996.
Shinoka, T. 2002. Artificial Organs. "Tissue Engineered Heat Valves: Autologous Cell Seeding on Biodegradable Polymer Scaffold." 26(5): 402-406.
Yow, K.H., et al. 2006. British Journal of Surgery. "Tissue engineering of vascular conduits." 93(6): 652-661.
Hao-Fan Peng, Jin Yu Liu, Stelios T. Andreadis, and Daniel D. Swartz. "Hair Follicle-Derived Smooth Muscle Cells and Small Intestinal Submucosa for Engineering Mechanically Robust and Vasoreactive Vascular Media." Tissue Engineering Part A. April 2011, 17(7-8): 981-990.
Stem Cell
Willenberg, B.J., et al. 2006. Journal of Biomaterials Res A. "Self-assembled copper-capillary alginate gel scaffolds with oligochitosan support embryonic stem cell growth." 79(2): 440-50.
M.J. Moreno, A. Ajji, D. Mohebbi-Kalhori, M. Rukhlova, A. Jadhizadeh, M.N. Bureau. Journal of Biomaterials Res B. "Development of a compliant and cytocompatible micro-fibrous polyethylene terephthalate vascular scaffold." Vol. 97B, Issue 2, pages 201-213, May 2011.
Scaffolds
Scheindler, M., et al. 2006. Cell Biochemistry and Biophysics. Living in three dimensions: 3D nano structured environments for cell culture and regenerative medicine. 45(2):215-27.
Zahir, N. and Weaver, V.M. 2004. Current Opinion in Genetics and Development Death in the third dimension: apoptosis regulation and tissue architecture.. 14(1): 71-80.
Zhang, S., et. al. 2005. Seminars in Cancer Biology. Designer self –assembling peptide nanofiber scaffolds for 3D tissue cell cultures. 15(5): 413-20.
Jones, D., et. al. 2009. A Versatile Approach to Scaffold Design for Bone in Growth Structures. Clinical Engineering, School of Clinical Sciences, University of Liverpool, UK
Drug Development
Andrei, G. 2006. Antiviral Research. Three-dimensional culture models for human viral diseases and antiviral drug development. 71(2-3): 96-107.