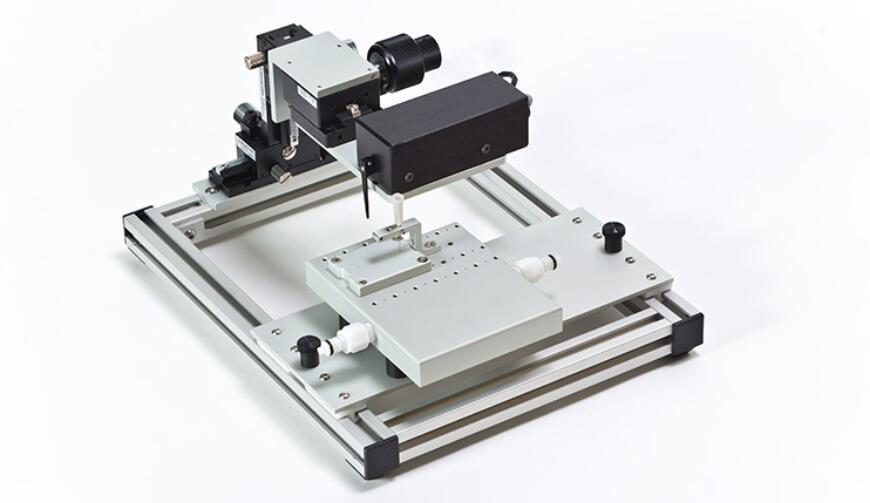
1300A: 3-in-1 Whole Animal System – Mouse
型号:1300A
价格:请致电:010-67529703
品牌:aurorascientific
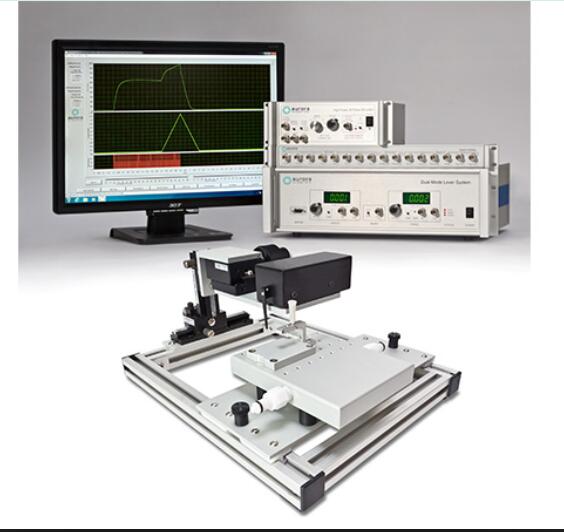
Overview
The 3-in-1 Whole Animal System provides flexible and accurate measurement of rodent muscle properties in-situ, in-vivo and in-vitro.
The 1300A and 1305A are high performing, precise test systems, giving researchers a simple method to test tensile and other mechanical properties of muscle. By combining in-situ, in-vivo and in-vitro muscle tests using one simple platform, researchers can capture a complete picture of muscle physiology in rodent subjects. The test systems come with either a mouse or rat apparatus, complete with temperature-controlled animal and limb plates designed to support and fix the animal and limb being tested. The system can be converted to an isolated (in-vitro) muscle test system with the attachment of an optional 25mL bath. Aurora Scientific’s flagship Dual-Mode lever system is also included, permitting measurement and control of both force and length.
Conveniently, muscle samples are attached at only one point to measure force and length, saving time and increasing productivity. This system also includes our high-power, bi-phasic stimulator and all required electrodes.
Control and analysis software comes pre-loaded on a custom PC, making the process of experimental setup, data collection and data analysis consistent and reliable.
With our control and analysis software (DMC/DMA), parameters such as resting length, resting force, stimulation and the actual test protocol are set and ready to use. An extensive library of standard experimental protocols such as twitch, tetanus, fatigue, force-frequency, force-velocity, stiffness and work loops are provided with the system.
Tell the Whole Story – Live Animal & Isolated Tissue in One
The three system configurations allows the researcher to work with a broad range of muscle types, providing a convenient platform for compound screening, phenotype evaluation and comparison of murine models; all within one system.
High Throughput Software Capability
Experience high-throughput data analysis, including batch processing and multi-parameter calculations for hundreds of muscle samples within minutes. Downstream analysis can be completed within Aurora Scientific DMC/DMA software or exported to your analysis program of choice.
Standard Protocol Library
The protocol library includes a variety of muscle experiments for rat in vitro studies. Protocols include system operation and data acquisition settings optimized for sample type and measurement needs. Add your own custom protocols as well to streamline system operation with multiple lab members.

Features
- powerful 3-in-1 design: save time and space while increasing productivity
- high experimental throughput
- fast data acquisition and analysis software for Windows or Linux operating systems
- dual purpose field/nerve stimulator
- ideal for live animal protocols: precision platform with temperature control
- convenience of one test system capable of studying both mice and rats
- range of peak forces from 0.5N to 10N
Case Studies
Several large, Fortune 500 pharmaceutical companies sought a way to quickly screen large groups of mice that had been treated with new experimental compounds/therapies. Existing equipment in house was either unavailable or difficult to use and lacked the capabilities needed to meet throughput demands. The precision of any in house equipment was also questionable.
1300A – 3-in-1 Test System Helps Major Pharmaceutical Companies Dramatically Improve Throughput
CHALLENGE

3-in-1 in-situ System Enables Biologist to Test Physiology of Muscle Regeneration
CHALLENGE
As a molecular and developmental biologist, Dr. Chen-Ming Fan of the Carnegie Institution for Science studies the molecular mechanisms involved in mammalian development, most notably in the musculoletal system. Over the years Dr. Fan has uncovered developmental mechanisms that are involved in muscle regeneration through the letal muscle stem cell niche, as regeneration is thought to be a recapitulation of development processes.
After identifying mechanisms that may aid in letal muscle regeneration, Dr. Fan needed a way to test if muscle function was improved or restored in aged and diseased mouse models.
SOLUTION
With the ability to test muscle function in-situ and in-vivo, the 1300A Whole Animal System was the logical choice for Dr. Fan to expand his research from molecular mechanisms to functional measurements. The in-situ setup would allow Dr. Fan to measure force produced by specific muscles in the hindlimb while keeping the muscle’s blood supply and nervous input intact. This represents a more physiological environment than the standard isolated muscle experiments. In addition, measurements of aggregate hindlimb function without surgery using the in-vivo setup would permit longitudinal study of muscle regeneration and functional recovery in aged and disease states.
RESULTS
Once the whole animal system was installed and demonstration complete, Dr. Fan and his students were extremely excited about the prospect of the system and began making functional measurements immediately. The lab employed the in-situ technique to measure functional recovery of the Tibialis Anterior (TA) following experimentally induced muscle injury in aged and dystrophic mice. Just over a year later, Dr. Fan’s lab published a paper in Nature Medicine citing our 1300A system. Dr. Fan stated it would not have been possible to publish this article without the 1300A whole animal system and he continues to utilize this powerful system to further test the mechanisms involved in muscle regeneration.

Citations
Rooney, Jachinta and Rich Lovering. “Single muscle contractile measurements in vivo and in situ.” NIHSOP: MDC1A_M.2.2.002 (2015): 1-8.
Al-Sajee, Dhuha et al. “Xin-deficient mice display myopathy, impaired contractility, attenuated muscle repair and altered satellite cell functionality.” Acta Physiologica 214.2 (2015): 248-260.
Thomas, Melissa M. et al. “Early oxidative shifts in mouse letal muscle morphology with high?fat diet consumption do not lead to functional improvements.” Physiological Reports 2.9 (2014): e12149.
Ambrosio, Fabrisia et al. “Arsenic induces sustained impairment of letal muscle and muscle progenitor cell ultrastructure and bioenergetics.” Free Radical Biology and Medicine 74 (2014): 64-73.
Distefano, Giovanna et al. “Neuromuscular Electrical Stimulation as a Method to Maximize the Beneficial Effects of Muscle Stem Cells Transplanted into Dystrophic letal Muscle.” PLoS ONE 8.3 (2013): e54922.
Alway, Stephen E. and Robert G. Cutlip. “Resistance Loading and Signaling Assays for Oxidative Stress in Rodent letal Muscle.” Methods in Molecular Biology Myogenesis (2011): 185-211.