美国 Shelfscientific,ISM-146NOXM,Nitric Oxide Measurement System (NO2/NO3),ArrowSTRAIGHT?氮氧化物测量系
ISM-146NOXM
请致电:010-67529703
美国 shelfscientific
ArrowSTRAIGHT?
Nitric Oxide Measurement System (NO2/NO3)
Your Straight Line to Accurate Results
ArrowSTRAIGHT?氮氧化物测量系
在zui小的样本中进行氮测试:血清、尿液、血液、唾液、组织匀浆、细胞培养

功能:不需要买昂贵的固定装置;
每个样本都节约在抗氧化剂方面的支出;
zui小样本制备步骤;
避免二氢烟酰胺腺嘌呤二核苷酸磷酸盐和抗氧化剂的干扰;
消除三氧化氮—还原步骤;
低检测限;
一氧化氮(NO)在多种哺乳动物的生化过程中起着重要的作用,其中包括血压稳态、免疫调节和神经系统信号传输方面。氮是非常不稳定的,它有一个生理半衰期1至40秒。即使用电化学分析法检测氮,对于其它的氮氧化合物而言,由于快速降解使它难以被高度鲸准地确定定量。氮的安培法实验是昂贵又浪费时间的,而且对运营商依赖性强。氮氧化后成为两种稳定的终级产品,亚硝酸盐(NO2-)和硝态氮(NO3-)离子。这两种终ji产品的浓度可以用来确定氮的产量,在测量过程中避免氮瞬间流失的问题。
有很多不同的方法可以定量测定亚硝酸盐(NO2-)和硝态氮(NO3-)离子浓度。zui常见的方法就是Griess与(NO2-)反应后产出一种含氮的的终ji产品。产出的终ji成品的颜色是紫色的。然后使用使用比色或分光光度分析技术将它量化。这个比色技术有三个基本的问题:shou先,抗氧化剂与(NO2-)反应会出现额外困难的一步,那就是完quan还原亚硝酸盐(NO2-)和硝态氮(NO3-)离子。其次,与锁有的比色法一样,Griess技术可以影响原始样本的颜色和浊度。zui后,这种方法是非常耗时和繁琐的,因为它涉及几个中级的化学反应。
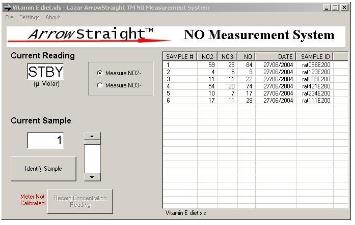
zui近,一个潜在的更准确,省时,简洁的方法已被开发用于测定NO2-和NO3-。这依赖于使用亚硝酸盐(NO2-)离子和硝态氮(NO3-)离子选择性电ji。这些电ji被制作得很小。将两种样本探测范围降到低微摩尔范围,这时电ji可以测量50—100微升的小样本。wu反应检测亚硝酸盐(NO2-)和硝态氮(NO3-)离子从而还原亚硝酸盐(NO2-)和硝态氮(NO3-)离子。由于这种方法被市场需求后,微型电ji系统变得更加完善。它通过RS232或USB端口连接到PC或笔记本对两种离子浓度进行复杂的离子分析和准确的测量。
规格:
微量亚硝酸盐离子电ji;
检测限:5微克分子
反应时间:45秒
zui小样本尺寸:50微升
硝酸盐离子电ji微
检测限:3微克分子
反应时间:45秒
zui小样本尺寸:50微升
Measure NO in Your Smallest Samples
- Plasma
- Serum
- Urine
- Whole blood
- Saliva
- Tissue homogenates
- Cell Cultures
EXCLUSIVE FEATURES:
- No expensive capital equipment to buy
- Per sample cost savings over Griess
- Minimize sample preparation steps
- Avoid interferences from NADPH and antioxidants (Griess)
- Eliminate NO3- reduction step (Griess)
- Low detection limits
Nitric oxide (NO) plays a major role in a variety of mammalian biological processes including blood pressure homeostasis, immune regulation, and nervous system signal transmission. NO is very unstable and has a physiological half life of only 1 to 40 seconds. Even though there are electrochemical (amperometric) methods for detecting NO, its rapid degradation to other nitrogen oxide compounds makes it difficult to determine quantitatively with a high degree of accuracy. The amperometric method for assay of NO is expensive, time consuming, and operator dependent. NO oxidizes into two stable end products, namely nitrite (NO2-) and nitrate (NO3-) ions. The concentration of these two end products can be used to quantify NO production without the measurement problems caused by the transient nature of NO.
There are various methods for quantitatively determining the concentration of both NO2- and NO3- ions. The most common methods involved the use of the Griess reagent which reacts with NO2- ion to produce a stable azo end product which is purple in color and can be quantified using colorimeteric or spectrophotometric analytical techniques. There are three basic problems with this colorimetric technique. First of all, the Griess reagent only reacts with the NO2- compound thereby requiring an additional difficult step to completely reduce NO3- ion to NO2-. Secondly, like with all colorimetric methods, the Griess technique can be affected by original sample color or turbidity. Thirdly, this method is very time consuming and tedious because it involved several intermediate chemical reactions.
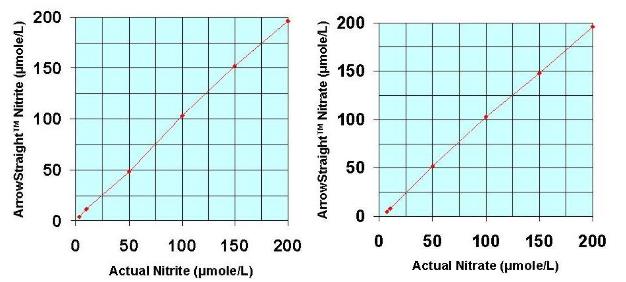
Recently a potentially more accurate, less time consuming, and less tedious method has been developed for assaying both NO2- and NO3- which relies on the use of ion selective electrodes for both NO2- and NO3- ions. These electrodes have been miniaturized to be able to measure samples down to 50 to 100 microliters with detection limits down to the low microMolar range for both species. Since both NO2- and NO3- ions can be measured individually no reaction to reduce NO3- to NO2- is required. This micro electrode system comes complete with a sophisticated ion analyzer which attaches via RS232 or USB port to a PC or laptop to give accurate concentration measurements for both ions.
Nitrite micro ion electrode
Detection limit: 5 microMolar
Response time: 45 seconds
Minimum sample size: 50 microliters
Nitrate micro ion electrode
Detection limit: 3 microMolar
Response time: 45 seconds
Minimum sample size: 50 microliters
Item number: ISM-146NOXM


