| 产品名称 | 3D组织器官芯片模型,SynBBB血脑屏障模型,Idealized Co-Culture Network Chips,Idealized Co-Culture Network Chips (IMN2 TEER),Idealized Co-Culture Network Chips (IMN2 Linear) |
| 品牌 | synvivo |
| 产品货号 | 3D组织器官芯片模型,SynBBB血脑屏障模型,Idealized Co-Culture Network Chips,Idealized Co-Culture Network Chips (IMN2 TEER),Idealized Co-Culture Network Chips (IMN2 Linear) |
| 产品价格 | 现货询价 |
| 联系人 | 李先生 |
| 联系电话 | 18618101725 |
产品说明3D组织器官芯片模型SynBBB血脑屏障模型,SynTumor 3D肿瘤模型,SynTox 3D毒理模型,SynRAM 3D炎症模型,synvivo血管模型芯片,SynALI气液界面肺模型二、SynBBB血脑屏障模型
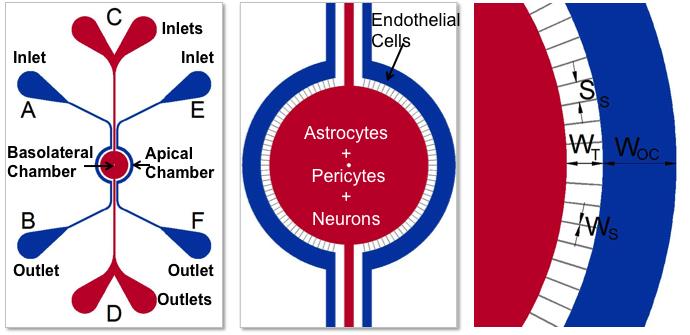
SynBBB血脑屏障模型通过复制脑组织细胞的组织学切片来重建体内微环境。该模型能够通过与跨血脑屏障(BBB)的内皮细胞进行通讯来实现此目的。结果,在SynBBB模型中,使用生理流体流很容易实现剪切诱导的内皮细胞紧密连接。这是其他模型(例如Transwell®模型)wu法实现的。紧密连接变化的形成可以使用SynVivo细胞阻抗分析仪通过生化或电气分析(评估电阻变化)进行测量。在SynBBB分析中,很容易看到大脑组织细胞与内皮细胞之间的相互作用。 Transwell®模型不允许实时显示这些细胞之间的相互作用,这对于理解BBB微环境至关重要。
用于开发BBB模型的设备的示意图。 顶腔(外通道)用于培养血管(内皮细胞),而基底外侧腔(中央腔)用于培养脑组织细胞(星形细胞,周细胞或神经元)。 多孔结构使血管细胞与组织细胞之间可以进行通讯。 外通道宽度(OC),行进宽度(T),狭缝间距(SS),狭缝宽度(WS)。
产品购买选项
SynVivo平台用于在芯片上创建个新生儿血脑屏障
SynVivo Platform Used to Develop Blood Tumor Barrier On-A-Chip
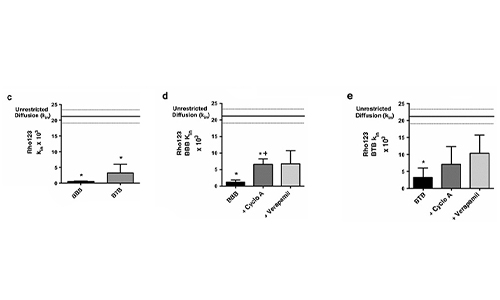
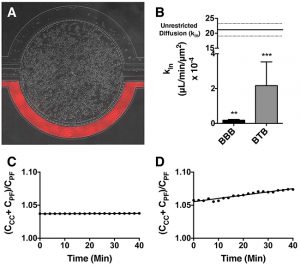
Permeability Across a Novel Microfluidic Blood-Tumor-Barrier Model In this study, Dr. Lockman’s team adapted the SynVivo BBB model to develop and characterize a BTB model. Results from the study demonstrate that the in vitro BTB model mimics the in vivo BTB with regard to permeability and efflux properties. In addition, inhibitor-based modulation of the permeability was readily quantified and compared very well with in vivo observations. According to Dr. Lockman “While some in vitro models have a flow component, this assay is the first-ever blood-tumor barrier developed using a commercially available microfluidic model with shear stress similar to that observed in vivo in addition to real-time visualization and quantitation.” This Blood Tumor Barrier model lays the foundation for use in screening assays for drug discovery and understanding of Central Nervous System diseases. Representative brightfield image of Rhodamine 123 dye accumulation in the central compartment after 90 min of perfusion in the BBB model without an inhibitor (a) and with an inhibitor (b). Rate of fluorescent dye accumulation of Rho123 into central compartment after 90 min of dye perfusion in BBB, and BTB chips (c). Rate of fluorescent dye accumulation in BBB (d) and BTB (e) chips perfused with Rho123 ± P-gp inhibitors (Cyclosporine A or Verapamil). Statistical significance was determined using one-way ANOVA followed by Tukey’s multiple comparison tests, and student’s t test; *p < 0.05 significance between tracer and unrestricted diffusion kin, n = 3–4; +p < 0.05 significance between BBB/BTB models and the addition of inhibitor, n = 3–6. All data represent mean ± SEM. White rectangle scale bars 500 μmSynBBB Blood Tumor Barrier On-A-Chip Advances Understanding of Therapeutic Transfer Across the Blood-Brain Barrier
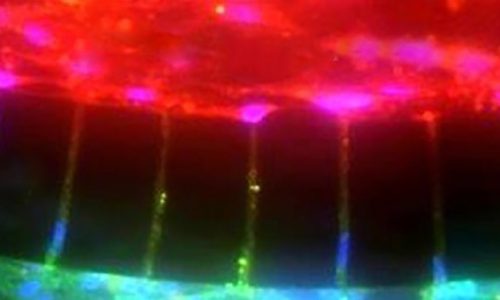
Trastuzumab Distribution in an In-Vivo and In-Vitro Model of Brain Metastases of Breast Cancer Researcher Paul Lockman and colleagues at West Virginia University, report on one of the first studies to monitor and quantify Trastuzumab (Herceptin®) movement across the blood-brain barrier using the SynVivo Blood-Brain Barrier (BBB) on-a-chip. Trastuzumab is a monoclonal antibody that is a widely used therapeutic for the treatment of HER2+ breast cancer. In the study published in Oncotarget titled “Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer”, SynVivo’s BBB model was adapted to develop a blood tumor barrier (BTB) model. The model was comprised of HER2+ breast cancer cells followed by real-time monitoring of the tissue distribution of the antibody trastuzumab. Data showed that the permeability of trastuzumab in-vivo increased from the BBB to the BTB similar to that observed in the SynVivo model. According to Dr. Lockman, “Development of an in vitro model with the ability to predict in vivo responses across the BBB is critical to progress our understanding and therapeutic strategies for brain disorders”. SynVivo’s in vivo validated microfluidic 3D tissue models provide a valuable resource for augmenting translational research for drug discovery and delivery. Mechanism of trastuzumab movement. Linear central compartment accumulation of t-Rho123 in in-vitro BBB and BTB microfluidic chip models. Representative image of model with TRITC labeled t-Rho123 flowing over HUVEC cells in the outer compartment and either astrocytes or JIMT-1 cancer cells in the central compartment (A). Rate of t-Rho123 movement in each model plotted against the unrestricted diffusion kin; ** p<0.0033 significance between BBB model and unrestricted diffusion kin, n=3; *** p<0.0005 significance between BTB model and unrestricted diffusion kin, n=3. All data represent mean ± S.E.M. Each model is significantly different than 0 (p < 0.05) (B). Representative graphs of the rate of accumulation of t-Rho123 in the BBB (C) and BTBSynBBB Blood-Brain Barrier On-A-Chip Assay Used For Screening of Novel Therapeutics in Real-Time
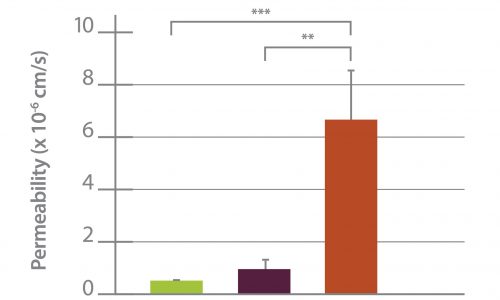
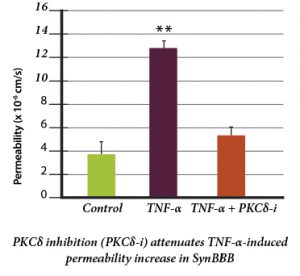
Protein Kinase C-Delta Inhibition Protects Blood-Brain Barrier from Sepsis-Induced Vascular Damage This publication reports on the use of the blood-brain barrier model to elucidate the regulation and relative contribution of Protein Kinase C-delta in the control of individual steps in neuroinflammation during sepsis. The role of PKC-delta-TAT peptide inhibitor as a potential therapeutic for the prevention or reduction of cerebrovascular injury in sepsis-induced vascular damage was also studied. Vascular integrity was assessed using the SynBBB Co-Culture model with primary human brain microvascular endothelial cells and Astrocytes. Endothelial cell permeability, TEER, and neutrophil transmigration were directly evaluated. SynBBB also allowed for real-time monitoring of neutrophil-endothelial interaction under physiologically relevant flow conditions. PKCδ activation is a key signaling event that alters the structural and functional integrity of BBB leading to vascular damage and inflammation-induced tissue damage. PKCδ-TAT peptide inhibitor has therapeutic potential for the prevention or reduction of cerebrovascular injury in sepsis-induced vascular damage. Human Blood-Brain Barrier on a Chip Developed with hCMEC/D3 Brain Endothelial Cell Line and Primary Human Astrocytes
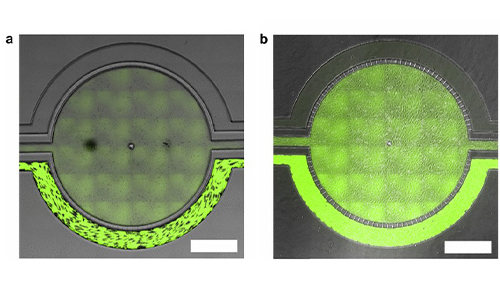
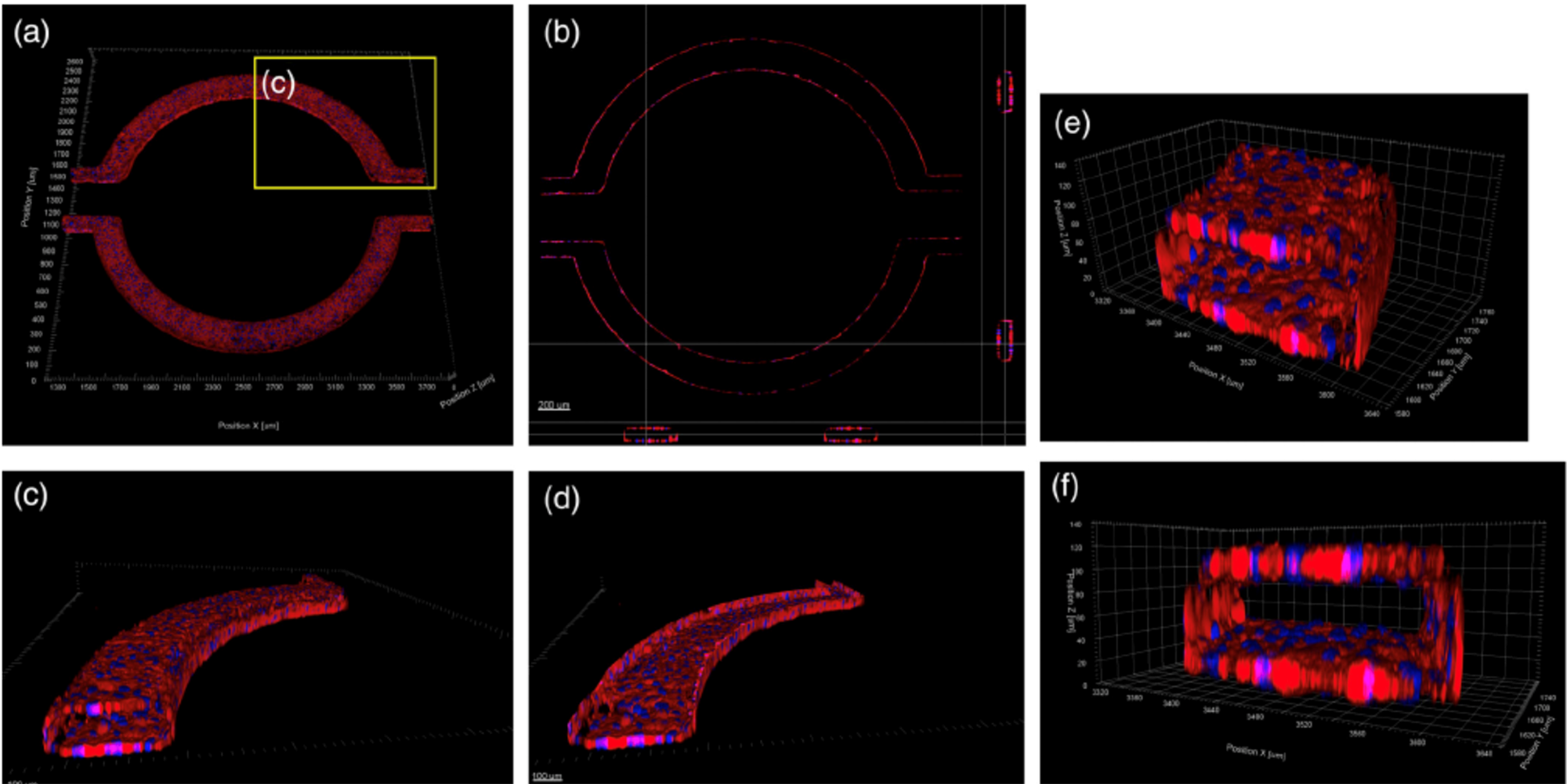
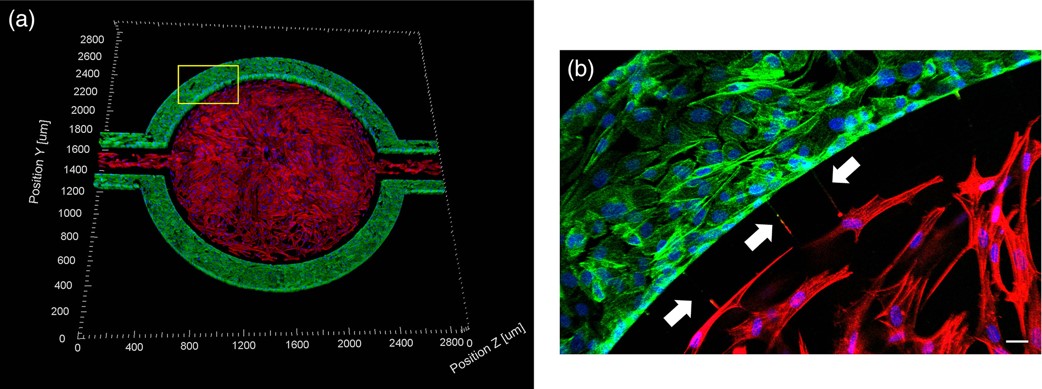
A Microfluidic Model of Human Brain (uHuB) for Assessment of Blood-Brain Barrier hCMEC/D3 cell line forms a complete lumen underflow and displays the appropriate tight junction markers. (a–f) Confocal images of hCMEC/D3 monolayers in the μHuB after conditioning to flow stained with ActinRed? 555 ReadyProbes? (actin, red) and Hoechst 33342 (nucleus, blue). (a) Onward‐looking view of μHuB device consisting of two vascular (apical) compartments lined with hCMEC/D3 monolayers. (b) Cross‐sectional view of hCMEC/D3 monolayers in μHuB forming a complete inner lumen approximately 200?μm (width) by 100?μm (height). (c) Onward‐looking view of one quadrant of the μHuB model as outlined in yellow in (a). (d) Lower half of (c), lined with a complete hCMEC/D3 monolayer. (e) Cross‐sectional view of inner lumen. (f) Same cross‐section as (e) at 90° viewing angleCo-Culture of hCMEC/D3 and Primary Astrocytes in the Human SynBBB Blood-Brain Barrier On-A-Chip hCMEC/D3 monolayers (green) were cultured in the vascular (apical) compartments with primary human astrocytes (red) in the tissue (basolateral) compartment (nuclei, blue). (a) Onward‐looking view of complete, three‐dimensional reconstruction of the co culture SynBBB model. (b) Zoomed‐in yellow region of (a) with arrows pointing to regions where astrocyte end‐feet are protruding to hCMEC/D3 monolayer. (scale bar for b?=?20?μm
| |