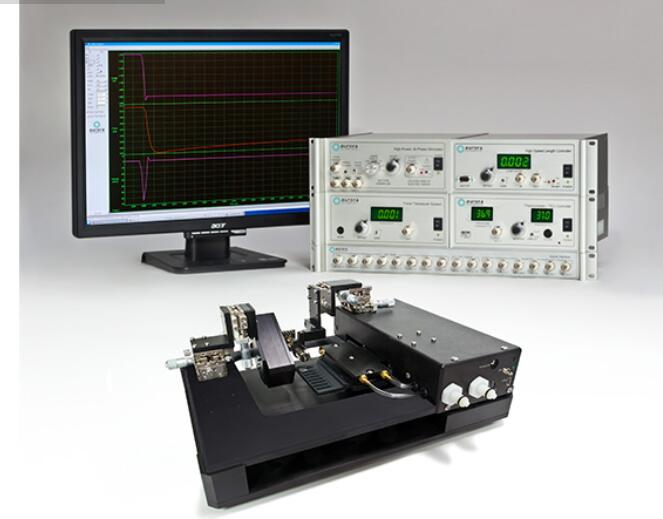
1400A: Permeabilized Fiber System – Microscope Mountable
型号:1400A
价格:请致电:010-67529703
品牌:aurorascientific
Overview
The 1400A and 1410A Permeabilized Fiber Systems are designed to enhance experimental throughput and simplify complex permeabilized fiber experiments. They provide accurate measurements of fiber properties across a broad range of applications and tests. Performing a force-pCa experiment is a breeze with our automatically indexing bath plate. Pre-program calcium concentrations and activation/relaxation sequences and let the 1400A system do the rest.
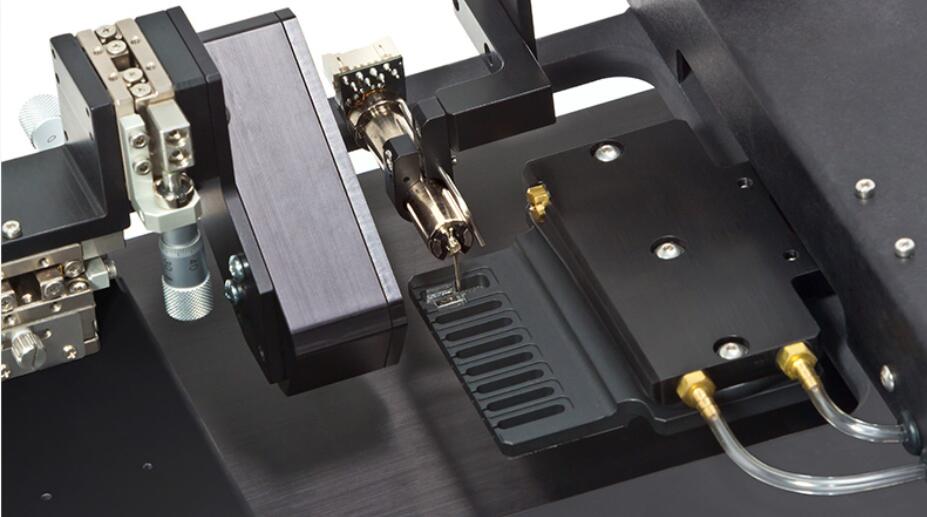
Included is temperature controlled apparatus which includes XYZ micrometer stages with built-in mounts for our high-speed length controllers and force transducers. The bath controller features exclusive software with a programmable motion control sequencer for automated bath transfer of the fiber being studied. Also included are a high-speed length controller, precision force transducer, data acquisition hardware and our unique real-time Linux control and analysis software.

Our dedicated software includes a library of experimental protocols, simplifying the process and allowing easy measurement of both force and length. When combined with our optional HVSL/VSL sarcomere length measurement system the researcher can control and measure length, force, and sarcomere length. These advanced features allow researchers to completely characterize permeabilized fibers performing all of the standard tests including force-pCa, kTr, length-tension, force-velocity and stiffness.
The Aurora Scientific permeabilized fiber test system is manufactured using corrosion resistant materials and can easily mount on an inverted microscope for basic observation or more sophisticated imaging.
Features
Case Studies
1400A – Durable System Helps Researcher Study Thousands of Fibers
CHALLENGE
In 2004, Dr. Hans Degens was studying changes in letal muscle function and morphology. Dr. Degens was looking to use permeabilized fibers as his test samples as they represent a pure system for studying cross bridge mechanics without bias and artifacts. Dr. Degens was familiar with the experimental methodology of using skinned fibers but lacked the resources to build a system from scratch that could handle the higher throughput demands of testing human samples.
SOLUTION
Aurora Scientific had recently released the 1400A system. This system featured an automatically indexing chamber enabling maximization of fiber throughput. The design also eliminated any manual bath movement, commonly found in designs at the time, thereby protecting the sensitive transducer from breakage. Dr. Degens’ system was also the first to feature peltier driven temperature control allowing for extremely stable and precise bath temperatures, critical to these measurements. The powerful 600A digital controller has allowed for nearly every experimental protocol conceived to be executed by the instruments themselves.
RESULTS
The system has been in nearly constant use in Dr. Degens’ lab for the last 11 years and has helped him collect data required for many influential and fascinating studies in exercise physiology, comparative biology and cardiovascular health, amongst countless others. This initial partnership with Aurora Scientific has led to other projects in whole muscle physiology as well.

Citations
Stoehr, Andrea, et al. “Automated analysis of contractile force and Ca2+ transients in engineered heart tissue.” American Journal of Physiology-Heart and Circulatory Physiology 306.9 (2014): H1353-H1363.
Shimkunas, Rafael, et al. “Myofilament dysfunction contributes to impaired myocardial contraction in the infarct border zone.” American Journal of Physiology-Heart and Circulatory Physiology 307.8 (2014): H1150-H1158.
Gineste, Charlotte, et al. “Alterations at the Cross-Bridge Level Are Associated with a Paradoxical Gain of Muscle Function In Vivo in a Mouse Model of Nemaline Myopathy.” PloS One 9.9 (2014): e109066.
Klaiman, Jordan M. “Cold acclimation increases cardiac myofilament function and ventricular pressure generation in trout.” The Journal of Experimental Biology 217 (2014): 4132-4140.
Bezold, Kristina L. et al. “A gain-of-function mutation in the M-domain of cardiac myosin-binding protein-C increases binding to actin.” Journal of Biological Chemistry 288.30 (2013): 21496-21505.
Kohn, Tertius A. and Timothy D. Noakes. “Lion (Panthera leo) and caracal (Caracal caracal) type IIx single muscle fibre force and power exceed that of trained humans.” The Journal of Experimental Biology 216.6 (2013): 960-969.
Lee, Eun-Jeong, et al. “Calcium sensitivity and myofilament lattice structure in titin N2B KO mice.” Archives of Biochemistry and Biophysics 535.1 (2013): 76-83.
Choi, Seung Jun et al. “Force-Generation Capacity of Single Vastus Lateralis Muscle Fibers and Physical Function Decline With Age in African Green Vervet Monkeys.” Journal of Gerontology Series A: Biological Sciences and Medical Sciences 68.3 (2013): 258-267.
Ochala, Julien and Lars Larsson. “Effects of a preferential myosin loss on Ca2+ activation of force generation in single human letal muscle fibres.” Experimental Physiology 93.4 (2008): 486-495.



