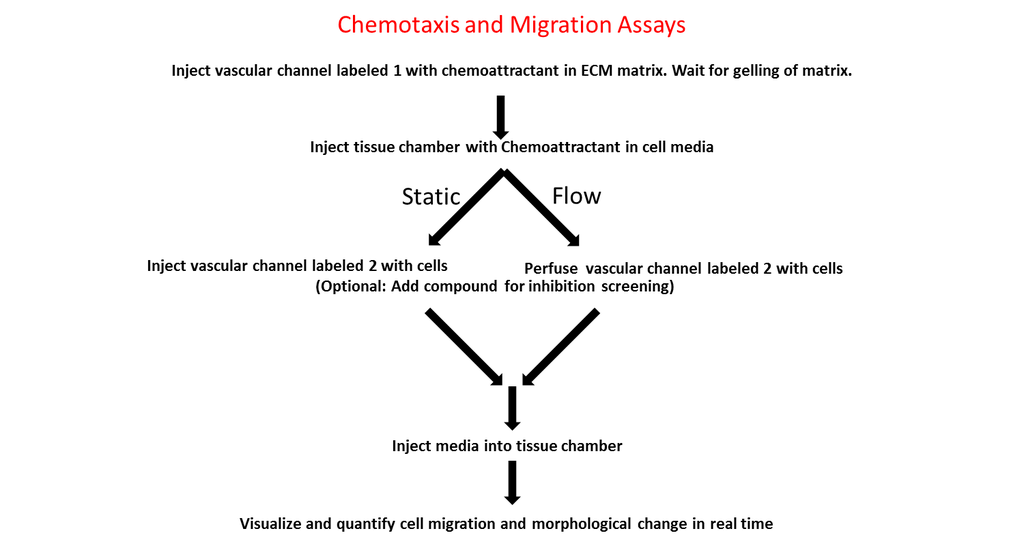
-
The SynRAM™ 3D Inflammation Model from SynVivo has been developed to study the entire inflammation pathway in a realistic and dynamic environment. By recreating a histological slice of co-cultured tissue and/or tumor cells with a lumen of endothelial cells, the SynVivo platform delivers a physiologically realistic model including flow and shear in a platform and enables real-time tracking of rolling, adhesion and migration processes. This model has been successfully validated against in vivo studies showing excellent correlation with rolling velocities, adhesion patterns and migratory processes (Lamberti et al 2014, Soroush et al 2016).
The SynRAM 3D inflammation model provides a realistic testing environment including:
Physiological shear stress within a microvascular environment
In vivo like vascular morphology with fully enclosed lumen
Co-culture capability for cell-cell interactions
Quantitative real-time rolling, adhesion, and migration data from a single experiment
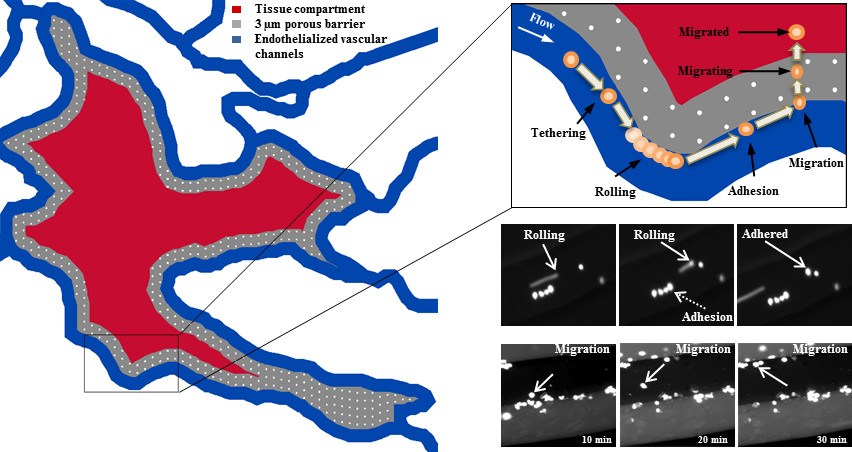
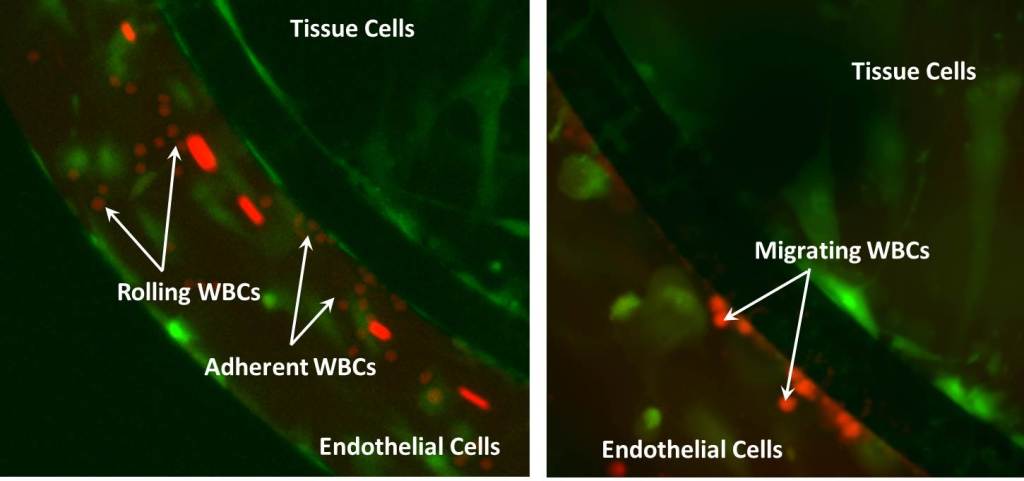
SynRAM enables assessment of cellular interactions comprising of rolling, adhesion and migration through multiple cellular layers in one experiment, in real-time, and represents data closely correlated with in vivo results.
SynRAM’s innovative design overcomes the current limitations inherent in flow chambers or Transwell chamber based assays. Current flow chamber designs are oversimplified, lack the scale and geometry of the microenvironment and cannot model transmigration. Similarly, Transwell chambers do not account for fluid shear and size/topology observed in vivo, the end point measurements of migration are not reproducible and do not provide real-time visualization.
SynVivo’s proprietary chip designs range from complex in vivo derived microvascular networks (obtained from digitized images) to produce realistic cellular makeup and vascular morphology resulting in varying shear and flow conditions, to simplified idealized networks designed to reproduce the cellular makeup and constant shear and flow conditions.
Idealized Network

Microvascular Network

SynRAM 3D Model Kit Components
All the basic components required to run assays using the the SynRAM model can be purchased in a kit format. Depending on individual research needs you can select from “idealized” or “microvascular” configurations of the SynRAM chip. All accessories including tubing, clamps, needles and syringes are included. Starter kits will also include the pneumatic priming device (required to run assays using SynRAM).
Kits include the following components:
Qty Description Cat# 10 SynRAM Chips (“idealized” or “microvascular”) 102008 or 105001 100ft Tygon Tubing (0.02″ ID x 0.06″ OD) 201005 25 Slide Clamps 202003 50 Blunt Tip Needles (24ga x 0.5″ long) 204002 50 1mL Syringes with Luer-Lock Tips 203004 1 SynVivo Pneumatic Primer Device (in starter kit only) 205001
SynRAM successfully validated against in vivo data
SynRAM microfluidic chips comprising of realistic microvascular networks were used to understand the role of classical inhibitors of individual steps of the leukocyte adhesion cascade. Experimental results matched very well with in vivo data highlighting the unique ability of the platform for real-time analysis of these dynamic events in a morphologically realistic environment (Lamberti et al 2014).
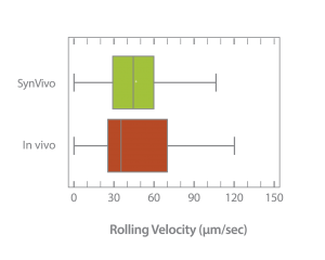
Neutrophil rolling using SynRAM microfluidic chips is similar to leukocyte rolling in vivo; Box and whisker plots summarizes the comparison of leukocytes rolling velocity measured in vivo and in SynRAM chips and shows no significant difference (p=0.758; Mann-Whitney Rank Sum Test). The “+” marked in the box indicates the mean.
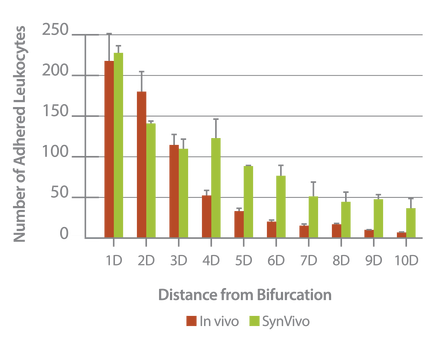
Neutrophil adhesion in SynRAM microfluidic chips is similar to leukocyte adhesion in vivo; Distribution of the number of adhered leukocytes and neutrophils as a function of distance from the nearest bifurcation in vivo in mouse cremaster muscle model and in vitro in microfluidic chips, respectively. Both histograms are skewed to the left indicating that leukocytes and neutrophils preferentially adhere near bifurcations with the peak occurring at one vessel or channel diameter from the nearest bifurcation.
Bioinspired Microfluidic Assay for In Vitro Modeling of Leukocyte–Endothelium Interactions. G. Lamberti, B. Prabhakarpandian, C. Garson, A. Smith, K. Pant, B. Wang, and M.F. Kiani. Anal. Chem., 2014, 86 (16), pp 8344–8351 DOI:10.1021/ac5018716
Investigation of the Effect of Blocking of Specific Steps of the Inflammation Pathway using Monoclonal Antibodies
Antibody blocking of specific steps in the adhesion/migration cascade downregulates other steps of the cascade; Monoclonal antibodies against E-selectin (aE-selectin), ICAM-1(aICAM-1), and PI3K (wortmannin) significantly reduced the number of rolling, adhering, and migrating neutrophils in SynRAM microfluidic devices.
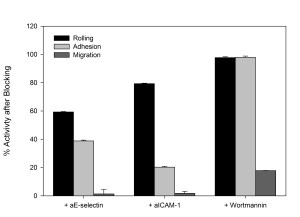
Percent activity after treating cells with the respective antibody blockers in comparison to their corresponding control values.
Elucidation of the Mechanism of Protein Kinase C delta (PKCδ) in Sepsis Related Inflammation Response
The SynRAM model was used to identify the underlying mechanism of Protein Kinase C delta (PKCδ) dependent neutrophil-endothelium interactions which has been found to play a significant role in the inflammatory response. Under physiological fluid flow conditions and using the simultaneous real-time monitoring ability of the entire inflammation process comprising of rolling, adhesion and migration, they found PKC𝛿 was a critical regulator of human neutrophil adhesion and migration through human endothelial cells during inflammation.
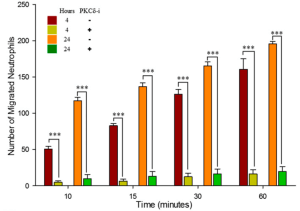
PKCδ-TAT inhibitor significantly reduces migration of neutrophils from the vascular channels, across the inflammed endothelium (treated with TNF-α for 4 or 24 hour), into the tissue compartment in response to fMLP mediated signaling compared to untreated controls.
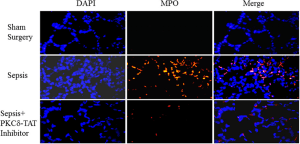
Immunohistochemical detection of myeloperoxidase (MPO) in representative lung tissue sections from 24 h post surgery. Few MPO-positive cells in Sham surgery. Sepsis induces the infiltration of numerous MPO-positive cells throughout the lung parenchyma. PKCδ-TAT Inhibitor significantly reduces sepsis-induced, MPO-positive cell numbers in the lung indicating decreased neutrophil migration.