细胞力学新型光镊,IMPETUX Optical Tweezers for Mechanobiology
型号: SENSOCELL
联系人:李胜亮
联系电话:18618101725
品牌:impetux
细胞力学新型光镊,IMPETUX Optical Tweezers for Mechanobiology
Impetux is a worldwide key supplier of turnkey optical tweezers systems designed for mechanobiology studies in living cells and 3D tissues.
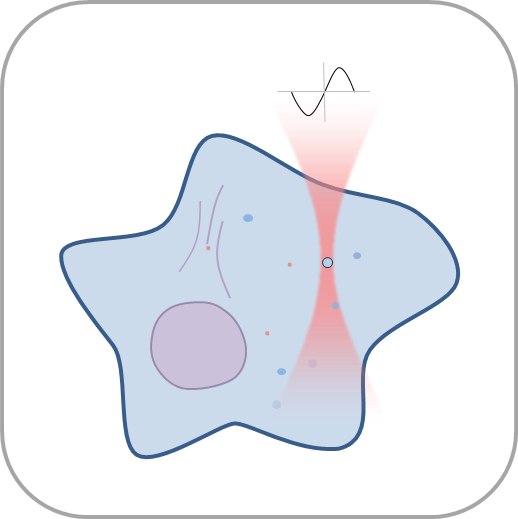
Mechanobiology is a growing topic that is bound to have a great impact on diverse research fields like cancer, morphogenesis, immunology or regenerative medicine. The optical tweezers platform SENSOCELL? enables outstanding and unprecedented optical trapping experiences for those researchers exploring new ways to boost their research:
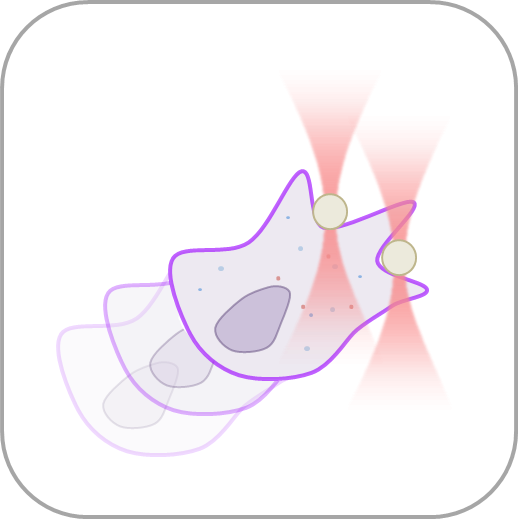
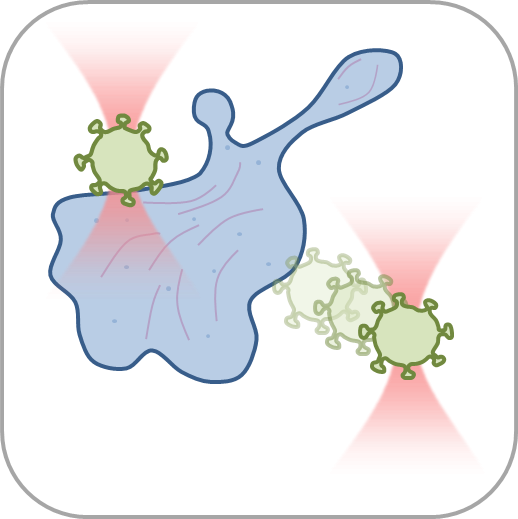
- Perform multiple optical trapping experiments with simultaneous force measurements and active/passive micro-rheology tests inside living cells or 3D tissues.
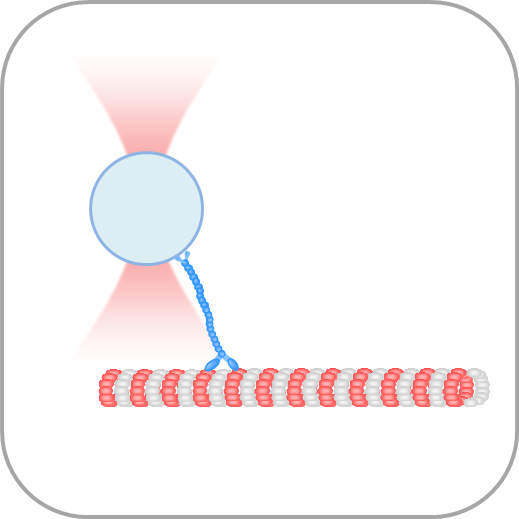
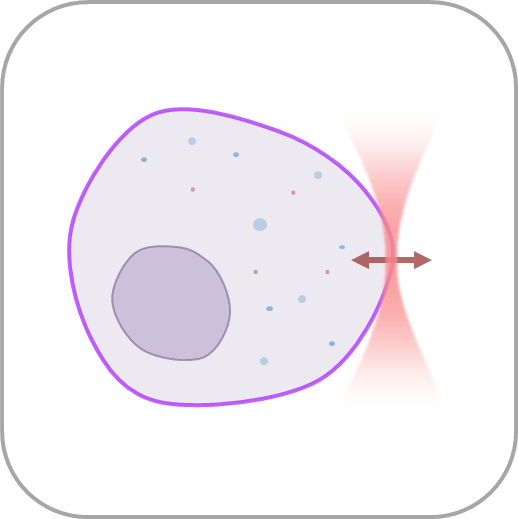
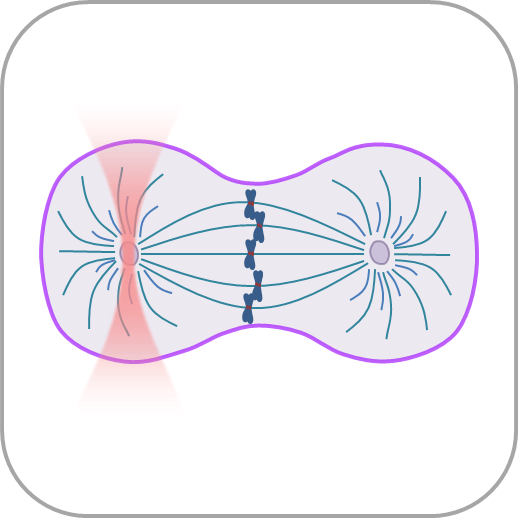
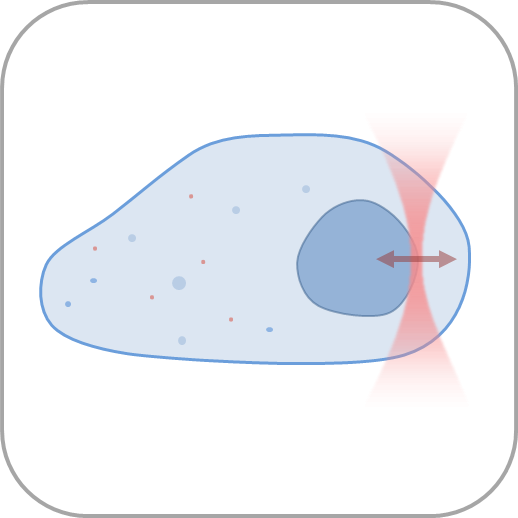
- Manipulate individual cells or cell organelles like nuclei, membranes or other native structures while tracking the forces involved in a non-invasive way.
Our worldwide patented technology not only allows measuring rheological properties and forces where others cannot but also gives unprecedented ease of use of optical tweezers for cell mechanics studies. Check the unique capabilities of our optical manipulation module Cygnium ? G-422 and force sensor Lunam ? T-40i to know more.
Papers:
- R. Meissner, N. Oliver and C.Denz. “Optical Force Sensing with Cylindrical Microcontainers“.Part. Part. Syst. Charact. 2018, 1800062.
-
F.Català, F. Marsà, M. Montes Usategui, A. Farré & E. Martín-Badosa. “Influence of experimental parameters on the laser heating of an optical trap“. Sci. Rep. 7, 16052; doi:10.1038/s41598-017-15904-6 (2017).
- Català, F. et al. “Extending calibration-free force measurements to optically-trapped rod-shaped samples“. Sci. Rep. 7, 42960; doi: 10.1038/srep42960 (2017).
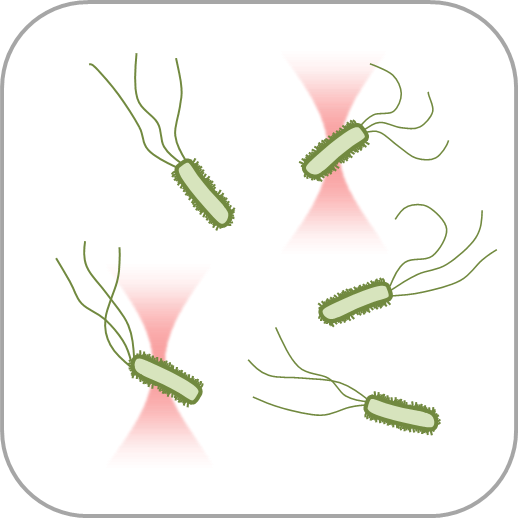
Optical trapping has become an optimal choice for biological research at the microscale due to its noninvasiveperformance and accessibility for quantitative studies, especially on the forces involved inbiological processes. However, reliable force measurements depend on the calibration of the opticaltraps, which is different for each experiment and hence requires high control of the local variables,especially of the trapped object geometry. Many biological samples have an elongated, rod-likeshape, such as chromosomes, intracellular organelles (e.g., peroxisomes), membrane tubules, certainmicroalgae, and a wide variety of bacteria and parasites. This type of samples often requires severaloptical traps to stabilize and orient them in the correct spatial direction, making it more difficult todetermine the total force applied. Here, we manipulate glass microcylinders with holographic opticaltweezers and show the accurate measurement of drag forces by calibration-free direct detection ofbeam momentum.
- R. Bola, F. Català. M. Montes-Usategui, E. Martín-Badosa. “Optical tweezers for force measurements and rheological studies on biological samples”.15th workshop on Information Optics (WIO), 2016.
Measuring forces inside living cells is still a challenge due the characteristics of the trapped organelles (non-spherical, unknown size and index of refraction) and the cell cytoplasm surrounding them heterogeneous and dynamic, non-purely viscous). Here, we show how two very recent methods overcome these limitations: on the one hand, forces can be measured in such environment by the direct detection of changes in the light momentum; on the other hand, an active-passive calibration technique provides both the stiffness of the optical trap as well as the local viscoelastic properties of the cell cytoplasm.
- Martín-Badosa, F. Català, J. Mas, M. Montes-Usategui, A. Farré, F. Marsà. “Force measurement in the manipulation of complex samples with holographic optical tweezers” 15th workshop on Information Optics (WIO), 2016.
-
Derek Craig, Alison McDonald, Michael Mazilu, Helen Rendall, Frank Gunn-Moore, and Kishan Dholakia. “ Enhanced Optical Manipulation of Cells Using Antireflection Coated Microparticles”.ACS Photonics, 2 (10), pp 1403–1409, (2015).
In molecular studies, an optically trapped bead may be functionalized to attach to a specific molecule, whereas in cell studies, direct manipulation with the optical field is usually employed. Using this approach, several methods may be used to measure forces with an optical trap. However, each has its limitations and requires an accurate knowledge of the sample parameters.6,7 In particular, force measurements can be challenging when working with nonspherical particles or in environments with an inhomogeneous viscosity, such as inside the cell. Recent developments in the field are moving toward obtaining direct force measurements by detecting light momentum changes. For this approach, the calibration factor only comes from the detection instrumentation and negates the requirement to recalibrate for changes in experimental conditions”.
-
Xing Ma, Anita Jannasch, Urban-Raphael Albrecht, Kersten Hahn, Albert Miguel-López, Erik Sch?ffer, and Samuel Sánchez. “Enzyme-Powered Hollow Mesoporous Janus Nanomotors”. Nano Lett., 15 (10), pp 7043–7050, (2015).
“Using optical tweezers, we directly measured a holding force of 64 ± 16 fN, which was necessary to counteract the effective self-propulsion force generated by a single nanomotor. The successful demonstration of biocompatible enzyme-powered active nanomotors using biologically benign fuels has a great potential for future biomedical applications.”
- Michael A. Taylor, Muhammad Waleed, Alexander B. Stilgoe, Halina Rubinsztein-Dunlop and Warwick P. Bowen. “Enhanced optical trapping via structured scattering“. Nature Photonics 9,669–673 (2015)
- Gregor Thalhammer, Lisa Obmascher, and Monika Ritsch-Marte, “Direct measurement of axial optical forces“.Optics Express, Vol. 23, Issue 5, pp. 6112-6129 (2015)
-
Y. Jun, S.K. Tripathy, B.R.J. Narayanareddy, M. K. Mattson-Hoss, S.P. Gross, “Calibration of Optical Tweezers for In Vivo Force Measurements: How do Different Approaches Compare?”. Biophysical Journal, V 107, 1474-1484 (2014).
Here, the authors present a comparison between two different methods for measuring forces inside living cells and provide measurements of the stall force of kinesin in vivo using the momentum-based approach. More information at: http://bioweb.bio.uci.edu/sgross/publications.html
- A. Farré, E. Martín-Badosa, and M. Montes-Usategui, “The measurement of light momentum shines the path towards the cell”, Opt. Pur Apl. 47, 239-248 (2014).
- A. Farré, F. Marsà, and M. Montes-Usategui, “A force measurement instrument for optical tweezers based on the detection of light momentum changes”, Proc. SPIE 9164, 916412 (2014).
- J. Mas, A. Farré, J. Sancho-Parramon, E. Martín-Badosa, and M. Montes-Usategui, “Force measurements with optical tweezers inside living cells”, Proc. SPIE 9164, 91640U (2014).
- F. Català, F. Marsà, A. Farré, M. Montes-Usategui, and E. Martín-Badosa, “Momentum measurements with holographic optical tweezers for exploring force detection capabilities on irregular samples”, Proc. SPIE 9164, 91640A (2014).
-
A. Farré, F. Marsà, and M. Montes-Usategui, “Optimized back-focal-plane interferometry directly measures forces of optically trapped particles” Opt. Express 20, 12270-12291 (2012).
This manuscript shows the relation between the determination of momentum measurements and back-focal-plane interferometry, and details how to obtain the force response of the sensor both from first principles and from its connection with trap stiffness calibration.
- A. Farré and M. Montes-Usategui, “A force detection technique for single-beam optical traps based on direct measurement of light momentum changes” Opt. Express 18, 11955-11968 (2010).
In this work, the authors show the feasibility of combining optical tweezers (single-beam gradient traps) with the determination of forces using the measurement of the light momentum change.