AIM BIOTECH 3D细胞培养芯片(AIM Biotech Microfluidic 3D Cell Culture Chips)
型号:3D Cell Culture Chips
价格:请致电:010-67529703
品牌:aim biotech/flexcell
AIM BIOTECH 3D细胞培养芯片概述
AIM的3D细胞培养芯片透气性好,而且用户可以通过选择不同的水凝胶,在间隔的3D和2D空间进行不同类型细胞的培养。同时可以通过对化学物浓度梯度和流体的调控很好地模拟符合用户特定需求的微环境。
1.多样化的聚合性凝胶填充
2.高效的气体交换
3.可控的流体及化学物浓度梯度
4.独特的共培养模型
· 特性:
1. 显微镜玻片尺寸 (75mm X 25mm)
2. 无菌 & 现成
3. 模块化设计,可利用AIM Luer Connectors开拓更多应用领域
4. 适用于386-孔板(AIM专有)
5. 底层所覆盖的是透光率高达92%的聚合物,因此该芯片可适用于相衬显微镜、荧光显微镜、2-photon & 共聚焦显微镜观察
6. 利用特殊的凹槽设计让培养基可以被快速地更换,即使使用真空抽吸器也不会有过度抽吸的风险
- Microscope slide format 75 mm X 25 mm.
- Compatible with all polymerisable gels including collagen, fibrinogen, Matrigel, etc. and combinations thereof.
- Gas permeable laminate for effective gas exchange.
- Optically clear and compatible with phase contrast, fluorescence and confocal microscopy.
- Enables monotypic or organotypic co-culture models.
- Enables the control of interstitial flow across the 3D gel region.
- Enables the control of chemical gradients across the 3D gel region.
- Sterile and ready-to-use.
- Designed for rapid media exchange through vacuum aspiration with no risk of over-aspiration.
- Designed for modular expansion with AIM Connectors.
- Fits into AIM Microtiter Plate Holders for easy handling and stacking.
Applications of AIM Biotech 3D Cell Culture Chips
- Cell migration of both adherent and non-adherent cells and cell invasion
- Angiogenesis and vasculogenesis
- Metastatic cancer assays (spheroid dispersion and extravasation)
- Long working regions that are easily injectable with hydrogel, with low risk of leakage.
- Gas permeable bottom laminate ensures accurate reflections of incubator conditions (normoxic or hypoxic).
- Multicellular co-culture, with meaningful organization into models of biological systems.
- Control over chemical gradients and flow across the gel region and/or within the media channels.
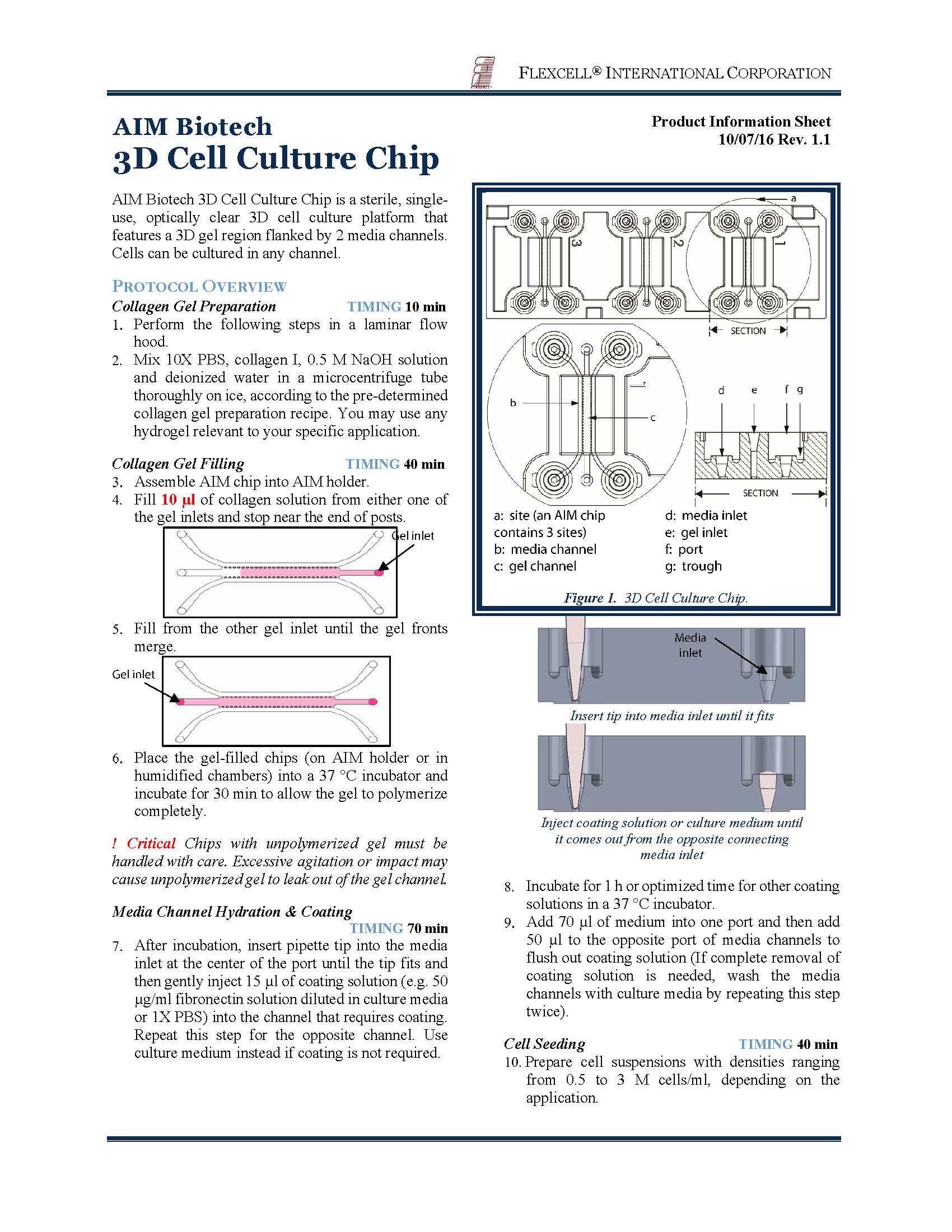
Novel posts enable easy gel filling & long working regions

|
AIM 3D Cell Culture Chips utilize a patented approach with a novel post design in conjunction with optimized post spacing & channel height. This allows hydrogels to be contained within gel channels during the hydrogel filling process, with little risk of leaking into adjacent channels. The DAX-1 chip, for instance, has a 14mm long gel region. The air-liquid interface is substantially flat & uniform, with minimal occurrence of concave (under-filled) or convex (over-filled) interfaces. As the hydrogel is caged within the gel channel, the meniscus that usually obstructs phase contrast imaging is also absent. In short, AIM chips make it easy for users to cast hydrogels for 3D cell culture, and provide excellent optical clarity for various imaging techniques. |
Gas permeable laminate

|
AIM 3D Cell Culture Chips are fabricated with gas permeable laminates to ensure that oxygen tension in each chip correctly reflects incubator conditions. Users have the flexibility of setting up normoxic or hypoxic culture conditions. |
Multicellular culture made possible, with meaningful organization into models of biological systems
 
|
The multi-channel design of AIM 3D Cell Culture Chips enables the co-culture of different cell types in distinct compartments in the device, yet allowing paracrine signalling between cell types to take place. The movement of cells between different channels (or within an individual channel) can be easily observed & tracked. The growth and/or migration of cells within gel can often cause gel shrinkage or degradation. This problem is mitigated by the use of posts in AIM chips. The posts help to stabilize the gel and increase cell culture duration before the matrix collapses. |
Control over chemical gradients & interstitial flow
 
|
A chemical concentration gradient can easily be created across the porous 3D hydrogel by using a higher concentration of the chemical in a channel and allowing diffusion to take place. This feature is very useful for studies where directional cues of effectors are critical, including angiogenesis, cell migration and neurite guidance. The interstitial flow across the 3D hydrogel can be controlled by setting up a pressure gradient between the flanking channels. This can be achieved by having a larger media volume in one media channel than the other, or by setting shear flow regimes that establish a pressure differential. AIM chips enable users to control shear flow in media channels with/without creating a pressure gradient across the gel channel. Shear flows are typically set by connecting the chip to a standard syringe pump through accessory connectors. |
Selected AIM Biotech Publications
- Vickerman V, Blundo J, Chung S and Kamm RD. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging Lab Chip, 2008, 8, 1468–1477, DOI: 10.1039/b802395f.
- Farahat W, Wood L, Zervantonakis I, Schor A, Ong S, Neal D, Kamm RD, and Asada H. Ensemble Analysis of Angiogenic Growth in Three-Dimensional Microfluidic Cell Cultures PLoS ONE 7(5): e37333. doi:10.1371/journal.pone.0037333, May 2012.
- Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, Kamm RD and Chung S. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nature Prot, 7(7):1247-1259, 2012, PMID: 22678430,
 AIM Biotech 3D Cell Culture Chip
AIM Biotech 3D Cell Culture Chip
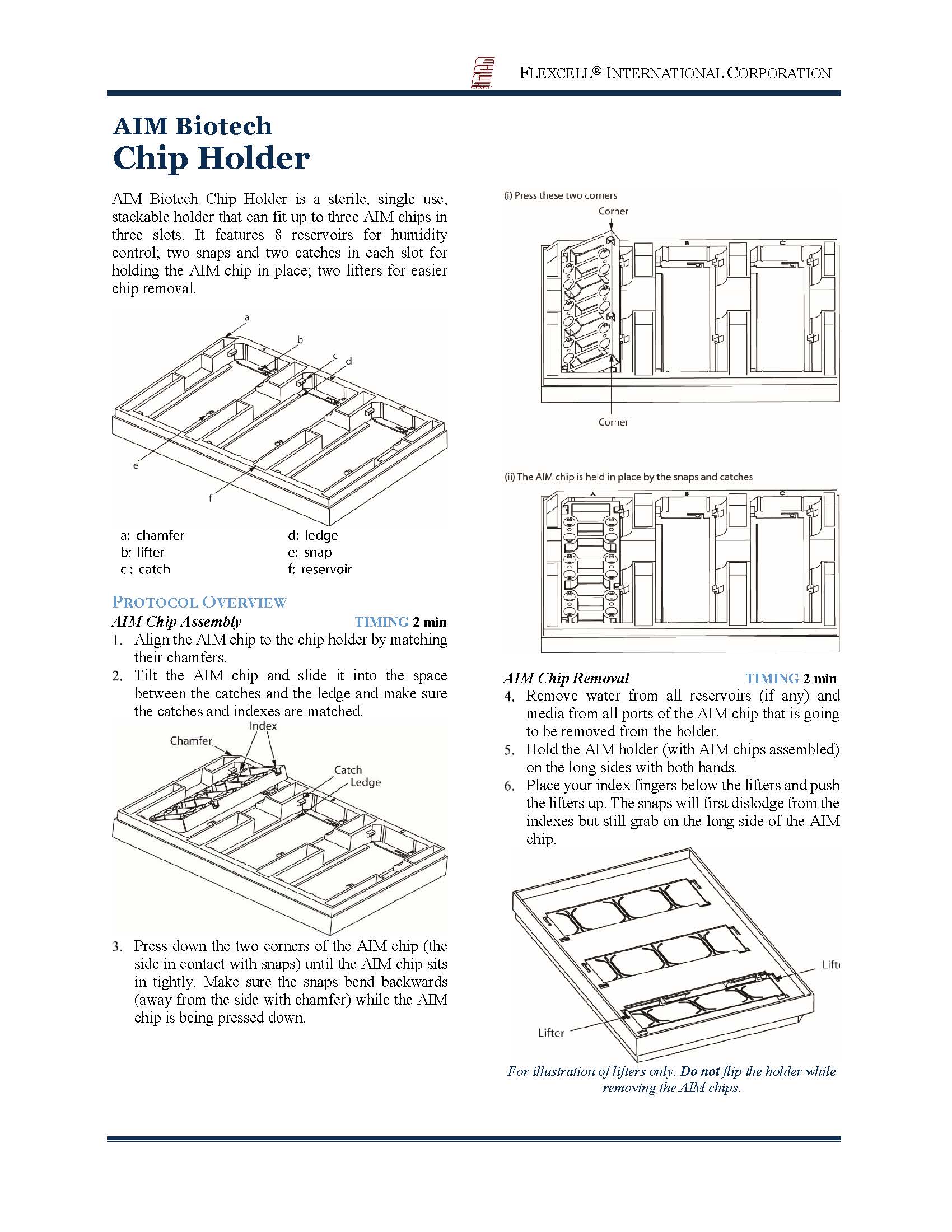
AIM Biotech Chip Holder

AIM Biotech Chip Holder

Technology
3D versus 2D cell culture
In vitro 3D cell culture models have emerged as a bridge between traditional 2D cell culture models and in vivo animal models. A carefully designed 3D model provides more physiologically relevant data for cell proliferation, morphology, dose response to drugs/toxins and gene expression profiles. This is because biological mechanisms that occur in 3D differ significantly from 2D culture in ways more consistent with in vivo observations [1-3]. The topographically complex 3D microenvironment accurately reflects in vivo conditions than a hard & flat plastic surface. Cell adhesion, cellular structure, effector transport and mechanotransduction are also substantially different in 3D systems [4]. This enables users to design experiments that previously could not be performed in conventional 2D assays, at a fraction of the cost of in vivo animal models.
|
Microfluidic devices for cell culture
Using microfluidic technologies for 3D cell culture brings additional benefits:
|
Key features of AIM microfluidic chips
- Long working regions that are easily injectable with hydrogel, with low risk of leakage
- Gas permeable bottom laminate ensures accurate reflections of incubator conditions (normoxic or hypoxic)
- Multicellular co-culture, with meaningful organization into models of biological systems
- Control over chemical gradients and flow across the gel region and/or within the media channels
Novel posts enable easy gel filling & long working regions
AIM 3D Cell Culture Chips utilize a patented approach with a novel post design in conjunction with optimized post spacing & channel height. This allows hydrogels to be contained within gel channels during the hydrogel filling process, with little risk of leaking into adjacent channels. The DAX-1 chip, for instance, has a 10.5mm long gel region.
The air-liquid interface is substantially flat & uniform, with minimal occurrence of concave (under-filled) or convex (over-filled) interfaces. As the hydrogel is caged within the gel channel, the meniscus that usually obstructs phase contrast imaging is also absent. In short, AIM chips make it easy for users to cast hydrogels for 3D cell culture, and provide excellent optical clarity for various imaging techniques. |
Multicellular culture made possible, with meaningful organization into models of biological systems
The multi-channel design of AIM 3D Cell Culture Chips enables the co-culture of different cell types in distinct compartments in the device, yet allowing paracrine signalling between cell types to take place. The movement of cells between different channels (or within an individual channel) can be easily observed & tracked.
The growth and/or migration of cells within gel can often cause gel shrinkage or degradation. This problem is mitigated by the use of posts in AIM chips. The posts help to stabilize the gel and increase cell culture duration before the matrix collapses. |
Control over chemical gradients & interstitial flow
A chemical concentration gradient can easily be created across the porous 3D hydrogel by using a higher concentration of the chemical in a channel and allowing diffusion to take place. This feature is very useful for studies where directional cues of effectors are critical, including angiogenesis, cell migration and neurite guidance.
|
|
The interstitial flow across the 3D hydrogel can be controlled by setting up a pressure gradient between the flanking channels. This can be achieved by having a larger media volume in one media channel than the other, or by setting shear flow regimes that establish a pressure differential.
AIM chips enable users to control shear flow in media channels with/without creating a pressure gradient across the gel channel. Shear flows are typically set by connecting the chip to a standard syringe pump through accessory connectors. |
Selected Publications
-
Vickerman V, Blundo J, Chung S and Kamm RD. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging Lab Chip, 2008, 8, 1468–1477, DOI: 10.1039/b802395f
- Farahat W, Wood L, Zervantonakis I, Schor A, Ong S, Neal D, Kamm RD, and Asada H. Ensemble Analysis of Angiogenic Growth in Three-Dimensional Microfluidic Cell Cultures PLoS ONE 7(5): e37333. doi:10.1371/journal.pone.0037333, May 2012.
- Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, Kamm RD and Chung S. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nature Prot, 7(7):1247-1259, 2012, PMID: 22678430
应用文献:
Publications
Many of the publications listed below were conducted on lab-made devices that form the basis of AIM Biotech chips. Papers that employed the commercial chips are marked with '*'.
|
|
Key Publications
- Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Vickerman V, Blundo J, Chung S, Kamm RD. Lab Chip, 2008, 8, 1468-1477.
- Cell migration into scaffold under co-culture conditions in a microfluidic platform. Chung S, Sudo S, Mack PJ, Wan C-R, Vickerman V, Kamm RD. Lab Chip, 2009, 9(2):269-75.
- Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, Kamm RD and Chung S. Nature Prot, 7(7):1247-1259, 2012, PMID: 22678430
- Mechanism of a flow-gated angiogenesis switch: early signaling events at cell-matrix and cell-cell junctions. Vickerman V, Kamm RD. Integr Biol (Camb). 2012 Jun 7. PMID 22722695
- Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13515-20. Epub 2012 Aug 6. PMID: 22869695
- Screening therapeutic EMT blocking agents in a three-dimensional microenvironment. Aref AR, Huang RY-J, Yu W, Chua K-N, Sun W, Tu T-Y, Sim W-J, Zervantonakis IK, Thiery JP, Kamm RD. Integr Biol (Camb). 2013 Feb;5(2):381-9. doi: 10.1039/c2ib20209c PMID: 23172153
- Mechanotransduction of fluid stresses governs 3D rheotaxis. Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD. Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2447-52. doi: 10.1073/pnas.1316848111. Epub 2014 Feb 3. PMID: 24550267
- Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Proceedings of the National Academy of Sciences, pp. 201417115, 2014
- A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as blood-brain barrier. Adriani G, Ma DL, Pavesi A, Kamm R, Goh ELK. Lab Chip, 2016. 17 (3):448-459
- *Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, ... Barbie DA. Cancer Discov. 2017 Nov 3. pii: CD-17-0833. doi: 10.1158/2159-8290.CD-17-0833.
1. Vascular Functions
1.1. Angiogenesis
- Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Vickerman V, Blundo J, Chung S, Kamm R. Lab Chip, 2008. 8 (9):1468-1477
- Surface-Treatment-Induced Three-Dimensional Capillary Morphogenesis in a Microfluidic Platform. Chung S, Sudo R, Zervantonakis IK, Rimchala T, Kamm RD. Advanced Materials, 2009. 21 (47):4863-4867
- Transport-mediated angiogenesis in 3D epithelial coculture. Sudo R, Chung S, Zervantonakis IK, Vickerman V, Toshimitsu Y, Griffith LG, Kamm RD. FASEB J., 2009. 23 (7):2155-2164
- Determining Cell Fate Transition Probabilities to VEGF/Ang 1 Levels: Relating Computational Modeling to Microfluidic Angiogenesis Studies. Das A, Lauffenburger D, Asada H, Kamm R. Cellular and Molecular Bioengineering, 2010. 3 (4):345-360
- Sprouting angiogenesis under a chemical gradient regulated by interactions with an endothelial monolayer in a microfluidic platform. Jeong GS, Han S, Shin Y, Kwon GH, Kamm RD, Lee SH, Chung S. Analytical Chemistry, 2011. 83 (22):8454-8459
- In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Shin Y, Jeon JS, Han S, Jung GS, Shin S, Lee SH, . . . Chung S. Lab Chip, 2011. 11 (13):2175-2181
- Ensemble Analysis of Angiogenic Growth in Three-Dimensional Microfluidic Cell Cultures. Farahat WA, Wood LB, Zervantonakis IK, Schor A, Ong S, Neal D, . . . Asada HH. PLoS ONE, 2012. 7 (5):e37333
- Engineering of In Vitro 3D Capillary Beds by Self-Directed Angiogenic Sprouting. Chan JM, Zervantonakis IK, Rimchala T, Polacheck WJ, Whisler J, Kamm RD. PLoS ONE, 2012. 7 (12):e50582
- Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, . . . Chung S. Nature Protocols, 2012. 7 (7):1247-1259
- In vitro angiogenesis assay for the study of cell-encapsulation therapy. Kim C, Chung S, Yuchun L, Kim M-C, Chan JKY, Asada HH, Kamm RD. Lab Chip, 2012. 12 (16):2942-2950
- Complementary effects of ciclopirox olamine, a prolyl hydroxylase inhibitor and sphingosine 1-phosphate on fibroblasts and endothelial cells in driving capillary sprouting. Lim SH, Kim C, Aref AR, Kamm RD, Raghunath M. Integr. Biol., 2013. 5 (12):1474-1484
1.2. Anti-Angiogenesis
- The stabilization effect of mesenchymal stem cells on the formation of microvascular networks in a microfluidic device. Yamamoto K, Tanimura K, Mabuchi Y, Matsuzaki Y, Chung S, Kamm RD, . . . Sudo R. J. Biomech. Sci. Eng., 2013. 8 (2):114-128
- Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sharghi-Namini S, Tan E, Ong L-LS, Ge R, Asada HH. Sci. Rep., 2014. 4:4031
- A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Kim C, Kasuya J, Jeon J, Chung S, Kamm RD. Lab Chip, 2015. 15 (1):301-310
1.3. Vasculogenesis
- Control of Perfusable Microvascular Network Morphology Using a Multiculture Microfluidic System. Whisler JA, Chen MB, Kamm RD. Tissue Engineering Part C: Methods, 2014. 20 (7):543-552
- In Vitro Microvessel Growth and Remodeling within a Three-Dimensional Microfluidic Environment. Park Y, Tu T-Y, Lim S, Clement IM, Yang S, Kamm R. Cellular and Molecular Bioengineering, 2014. 7 (1):15-25
- Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, . . . Kamm RD. Integr. Biol., 2014. 6 (5):555-563
- Human Vascular Tissue Models Formed from Human Induced Pluripotent Stem Cell Derived Endothelial Cells. Belair DG, Whisler JA, Valdez J, Velazquez J, Molenda JA, Vickerman V, . . . Murphy WL. Stem Cell Reviews and Reports, 2015. 11 (3):511-525
- Elucidation of the Roles of Tumor Integrin β1 in the Extravasation Stage of the Metastasis Cascade. Chen MB, Lamar JM, Li R, Hynes RO, Kamm RD. Cancer Res., 2016. 76 (9):2513-2524
- On-chip human microvasculature assay for visualization and quantitation of tumor cell extravasation dynamics. Chen MB, Whisler JA, Fr?se J, Yu C, Shin YJ and Kamm RD. Nat Protoc. 2017 May; 12(5): 865–880.
- *Functional human 3D microvascular networks on a chip to study the procoagulant effects of ambient fine particulate matter. Li Y, Pi QM, Wang PC, Liu LJ, Han ZG, Shao Y, Zhai Y, Zuo ZY, Gong ZY, Yang X, Yang W. RSC Adv., 2017, 7, 56108–56116
1.4. Flow Response
- Mechanism of a flow-gated angiogenesis switch: Early signaling events at cell-matrix and cell-cell junctions. Vickerman V, Kamm RD. Integr. Biol., 2012. 4 (8):863-874
1.5. Transendothelial Migration
- A versatile assay for monitoring in vivo-like transendothelial migration of neutrophils. Han S, Yan JJ, Shin Y, Jeon JJ, Won J, Jeong HE, . . . Chung S. Lab Chip, 2012. 12 (20):3861-3865
1.6. Migration
- Vascular Endothelial Growth Factor (VEGF) and Platelet (PF-4) Factor 4 Inputs Modulate Human Microvascular Endothelial Signaling in a Three-Dimensional Matrix Migration Context. Hang T-C, Tedford NC, Reddy RJ, Rimchala T, Wells A, White FM, . . . Lauffenburger DA. Molecular & Cellular Proteomics : MCP, 2013. 12 (12):3704-3718
- Cell Invasion Dynamics into a Three Dimensional Extracellular Matrix Fibre Network. Kim M-C, Whisler J, Silberberg YR, Kamm RD, Asada HH. PLoS Comput Biol, 2015. 11 (10):e1004535
1.7. Permeability
- Constructive remodeling of a synthetic endothelial extracellular matrix. Han S, Shin Y, Jeong HE, Jeon JS, Kamm RD, Huh D, . . . Chung S. Sci. Rep., 2015. 5:18290
2. Cancer Biology
2.1. Spheroid Dispersion
- Screening therapeutic EMT blocking agents in a three-dimensional microenvironment. Aref AR, Huang RY-J, Yu W, Chua K-N, Sun W, Tu T-Y, . . . Kamm RD. Integr. Biol., 2013. 5 (2):381-389
- Validating Antimetastatic Effects of Natural Products in an Engineered Microfluidic Platform Mimicking Tumor Microenvironment. Niu Y, Bai J, Kamm RD, Wang Y, Wang C. Mol. Pharm., 2014. 11 (7):2022-2029
- Inhibition of KRAS-driven tumorigenicity by interruption of an autocrine cytokine circuit. Zhu Z, Aref AR, Cohoon TJ, Barbie TU, Imamura Y, Yang S, . . . Barbie DA. Cancer Discov., 2014. 4 (4):452-465
- Targeting an IKBKE cytokine network impairs triple-negative breast cancer growth. Barbie TU, Alexe G, Aref AR, Li S, Zhu Z, Zhang X, . . . Gillanders WE. The Journal of Clinical Investigation, 2014. 124 (12):5411-5423
- Development of covalent inhibitors that can overcome resistance to first-generation FGFR kinase inhibitors. Tan L, Wang J, Tanizaki J, Huang Z, Aref AR, Rusan M, . . . Gray NS. Proc. Natl. Acad. Sci. USA, 2014. 111 (45):E4869-E4877
- Identification of drugs as single agents or in combination to prevent carcinoma dissemination in a microfluidic 3D environment. Bai J, Tu T-Y, Kim C, Thiery JP, Kamm RD. Oncotarget, 2015. 6 (34):36603-36614
- Contact-dependent carcinoma aggregate dispersion by M2a macrophages via ICAM-1 and β2 integrin interactions. Bai J, Adriani G, Dang T-M, Tu T-Y, Penny H-XL, Wong S-C, . . . Thiery J-P. Oncotarget, 2015. 6 (28):25295-25307
- *Stimuli-Responsive Nanodiamond-Based Biosensor for Enhanced Metastatic Tumor Site Detection. Wang X, Gu MJ, Toh TB, Abdullah NLB, Chow E. SLAS Technol. 2018 Feb;23(1):44-56. doi: 10.1177/2472630317735497. Epub 2017 Oct 11.
2.2. Extravasation
- In Vitro Model of Tumor Cell Extravasation. Jeon JS, Zervantonakis IK, Chung S, Kamm RD, Charest JL. PLoS ONE, 2013. 8 (2):e56910
- Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Chen MB, Whisler JA, Jeon JS, Kamm RD. Integr. Biol., 2013. 5 (10):1262-1271
- A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, . . . Kamm RD. Biomaterials, 2014. 35 (8):2454-2461
- Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Proc. Natl. Acad. Sci. USA, 2015. 112 (1):214-219
- Neutrophils suppress intraluminal NK-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, . . . Weinberg RA. Cancer Discov., 2016. 6 (6):630-649
- Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Penny HL, Sieow JL, Adriani G, Yeap WH, See Chi Ee P, San Luis B, . . . Wong SC. OncoImmunology, 2016. 5 (8):e1191731
- On-chip human microvasculature assay for visualization and quantitation of tumor cell extravasation dynamics. Chen MB, Whisler JA, Fr?se J, Yu C, Shin YJ and Kamm RD. Nat Protoc. 2017 May; 12(5): 865–880.
2.3. Intravasation
- Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Proc. Natl. Acad. Sci. USA, 2012. 109 (34):13515-13520
2.4. Flow Response
- Interstitial flow influences direction of tumor cell migration through competing mechanisms. Polacheck WJ, Charest JL, Kamm RD. Proc. Natl. Acad. Sci. USA, 2011. 108 (27):11115-20
- Mechanotransduction of fluid stresses governs 3D cell migration. Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD. Proc. Natl. Acad. Sci. USA, 2014. 111 (7):2447-2452
2.5. Invasion and Migration
- Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD. Lab Chip, 2009. 9 (2):269-275
- Concentration gradients in microfluidic 3D matrix cell culture systems. Zervantonakis I, Chung S, Sudo R, Zhang M, Charest J, Kamm R. International Journal of Micro-Nano Scale Transport, 2010. 1 (1):27-36
- A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Funamoto K, Zervantonakis IK, Liu Y, Ochs CJ, Kim C, Kamm RD. Lab Chip, 2012. 12 (22):4855-4863
- Hydrogels: Extracellular Matrix Heterogeneity Regulates Three-Dimensional Morphologies of Breast Adenocarcinoma Cell Invasion. Shin Y, Kim H, Han S, Won J, Jeong HE, Lee E-S, . . . Chung S. Advanced Healthcare Materials, 2013. 2 (6):920-920
- A three-dimensional microfluidic tumor cell migration assay to screen the effect of anti-migratory drugs and interstitial flow. Kalchman J, Fujioka S, Chung S, Kikkawa Y, Mitaka T, Kamm RD, . . . Sudo R. Microfluid. Nanofluid., 2013. 14 (6):969-981
- Breast Cancer Cell Invasion into a Three Dimensional Tumor-Stroma Microenvironment. Truong D, Puleo J, Llave A, Mouneimne G, Kamm RD, Nikkhah M. Sci. Rep., 2016. 6:34094
- Macrophage-secreted TNFα and TGFβ1 Influence Migration Speed and Persistence of Cancer Cells in 3D Tissue Culture via Independent Pathways. Li R, Hebert JD, Lee TA, Xing H, Boussommier-Calleja A, Hynes RO, . . . Kamm RD. Cancer Res., 2016. 77 (2):279-290
2.6. Testing New Therapeutic Approaches
- Engineering a 3D microfluidic culture platform for tumor-treating field application. Pavesi A, Adriani G, Tay A, Warkiani ME, Yeap WH, Wong SC, Kamm RD. Sci. Rep., 2016. 6:26584
3. Immunotherapy
- *A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. Pavesi A, Tan AT, Koh S, Chia A, Colombo M, Antonecchia E, Miccolis C, Ceccarello E, Adriani G, Raimondi MT, Kamm RD, Bertoletti A. JCI Insight. 2017 Jun 15;2(12). pii: 89762. doi: 10.1172/jci.insight.89762.
- *Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, ... Barbie DA. Cancer Discov. 2017 Nov 3. pii: CD-17-0833. doi: 10.1158/2159-8290.CD-17-0833.
- *CDK4/6 Inhibition Augments Anti-Tumor Immunity by Enhancing T Cell Activation. Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, ... Wong KK. Cancer Discov. 2017 Nov 3. pii: CD-17-0915. doi: 10.1158/2159-8290.CD-17-0915.
- Characterizing the Role of Monocytes in T Cell Cancer Immunotherapy Using a 3D Microfluidic Model. Lee SWL, Adriani G, Ceccarello E, Pavesi A, Tan AT, Bertoletti A, Kamm RD and Wong SC (2018) Front. Immunol. 9:416. doi: 10.3389/ mmu.2018.00416
4. Neurobiology
- A high-throughput microfluidic assay to study neurite response to growth factor gradients. Kothapalli CR, van Veen E, de Valence S, Chung S, Zervantonakis IK, Gertler FB, Kamm RD. Lab Chip, 2011. 11 (3):497-507
- A microfluidic device to investigate axon targeting by limited numbers of purified cortical projection neuron subtypes. Tharin S, Kothapalli CR, Ozdinler PH, Pasquina L, Chung S, Varner J, . . . Macklis JD. Integr. Biol., 2012. 4 (11):1398-1405
- Three-dimensional extracellular matrix-mediated neural stem cell differentiation in a microfluidic device. Han S, Yang K, Shin Y, Lee JS, Kamm RD, Chung S, Cho SW. Lab Chip, 2012. 12 (13):2305-2308
- A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as blood-brain barrier. Adriani G, Ma DL, Pavesi A, Kamm R, Goh ELK. Lab Chip, 2016. 17 (3):448-459
5. Stem Cell Biology
5.1. Differentiation of Embryonic Stem Cells
- Differentiation of embryonic stem cells into cardiomyocytes in a compliant microfluidic system. Wan CR, Chung S, Kamm RD. Ann. Biomed. Eng., 2011. 39 (6):1840-1847
- Simultaneous or Sequential Orthogonal Gradient Formation in a 3D Cell Culture Microfluidic Platform. Uzel SGM, Amadi OC, Pearl TM, Lee RT, So PTC, Kamm RD. Small, 2016. 12 (5):612-622
5.2. Electrical and Mechanical Stimulation of Mesenchymal Stem Cells
- Controlled electromechanical cell stimulation on-a-chip. Pavesi A, Adriani G, Rasponi M, Zervantonakis IK, Fiore GB, Kamm RD. Sci. Rep., 2015. 5:11800
6. Mechanobiology
6.1. Mechanical stimulation of Cardiac Fibroblasts
- On-chip assessment of human primary cardiac fibroblasts proliferative responses to uniaxial cyclic mechanical strain. Ugolini GS, Rasponi M, Pavesi A, Santoro R, Kamm R, Fiore GB, . . . Soncini M. Biotechnol. Bioeng., 2016. 113 (4):859-869
6.2. Optically Excitable Motor Units
- Microfluidic device for the formation of optically excitable, three-dimensional, compartmentalized motor units. Uzel SGM, Platt RJ, Subramanian V, Pearl TM, Rowlands CJ, Chan V, . . . Kamm RD. Science Advances, 2016. 2 (8)
7. Other Models
7.1. Environmental Assessment
- *Functional human 3D microvascular networks on a chip to study the procoagulant effects of ambient fine particulate matter. Li Y, Pi QM, Wang PC, Liu LJ, Han ZG, Shao Y, Zhai Y, Zuo ZY, Gong ZY, Yang X, Yang W. RSC Adv., 2017, 7, 56108–56116
- *Protein corona of airborne nanoscale PM2.5 induces aberrant proliferation of human lung fibroblasts based on a 3D organotypic culture. Li Y, Wang PC, Hu CL, Wang K, Chang Q, Liu LJ, Han ZG, Shao Y, Zhai Y, Zuo ZY, Gong ZY, Wu Y. Scientific Reports volume 8, Article number: 1939(2018) doi:10.1038/s41598-018-20445-7
8. Reviews
- Microfluidic Platforms for Studies of Angiogenesis, Cell Migration, and Cell–Cell Interactions. Chung S, Sudo R, Vickerman V, Zervantonakis IK, Kamm RD. Ann. Biomed. Eng., 2010. 38 (3):1164-1177
- Microfluidic devices for studying heterotypic cell-cell interactions and tissue specimen cultures under controlled microenvironments. Zervantonakis IK, Kothapalli CR, Chung S, Sudo R, Kamm RD. Biomicrofluidics, 2011. 5 (1)
- Microfluidic models of vascular functions. Wong KHK, Chan JM, Kamm RD, Tien J. 2012. 14:205-230
- Tumor cell migration in complex microenvironments. Polacheck WJ, Zervantonakis IK, Kamm RD. Cell. Mol. Life Sci., 2013. 70 (8):1335-1356
- Microfluidic platforms for mechanobiology. Polacheck WJ, Li R, Uzel SGM, Kamm RD. Lab Chip, 2013. 13 (12):2252-2267
- Creating living machines. Kamm RD, Bashir R. Ann. Biomed. Eng., 2014. 42 (2):445-459
- In vitro models of the metastatic cascade: from local invasion to extravasation. Bersini S, Jeon JS, Moretti M, Kamm RD. Drug Discov. Today, 2014. 19 (6):735-742
- Microfabrication and microfluidics for muscle tissue models. Uzel SGM, Pavesi A, Kamm RD. Progress in Biophysics and Molecular Biology, 2014. 115 (2–3):279-293
- Single-Cell Migration in Complex Microenvironments: Mechanics and Signaling Dynamics. Mak M, Spill F, Kamm RD, Zaman MH. J. Biomech. Eng., 2016. 138 (2):021004-021004-8
- Impact of the physical microenvironment on tumor progression and metastasis. Spill F, Reynolds DS, Kamm RD, Zaman MH. Curr. Opin. Biotechnol., 2016. 40:41-48
- Microfluidics: A New Tool for Modeling Cancer-Immune Interactions. Boussommier-Calleja A, Li R, Chen MB, Wong SC, Kamm RD. Trends in Cancer. 2 (1):6-19
- Microfluidic models for adoptive cell-mediated cancer immunotherapies. Adriani G, Pavesi A, Tan AT, Bertoletti A, Thiery JP, Kamm RD. Drug Discov. Today, 2016. 21 (9):1472-1478
- M2a macrophages induce contact-dependent dispersion of carcinoma cell aggregates. Adriani G, Bai J, Wong SC, Kamm RD, Thiery JP. Macrophage, 2016. 3:e1222







