|
DIMSCAN 半自动荧光数字图像显微处理系统
A New Generation of Fluorescence Digital Image Microscopy System for Measuring Cytotoxicity in Microplate
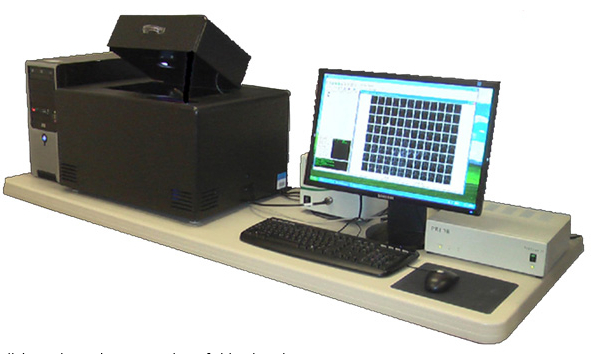
图1 DIMSCAN观测系统外观
2、系统亮点概述
DIMSCAN是用于在组织培养板中定量的相对细胞数半自动数字图像显微术系统。使用荧光素二乙酸酯(FDA)通过DIMSCAN测定细胞毒性测定法中,染料在活细胞选择性地累积,利用集落形成测定法,可以实现动态范围在4至7天内的生长曲线。
(1)活细胞的相对数目是通过测算荧光素二乙酸酯(FDA)的荧光强度,削减由背景荧光的阈值和由曙红所终止的不可存活的细胞的荧光值进行测量。
(2)可以使用含6至384个孔的矩形培养板,对于96孔板平均扫描时间为6分钟。
(3)系统采用了基于自相关函数的自动聚焦功能以最大限度地提高了系统的自动化的程度。
(4)相关程序存储重新扫描过程中的图像,并在用户扫描结束时显示出来,至今进行新的扫描过程。
(5)具有操作简便、准确、灵敏、重复性好等点,细胞数量在1, 10, 20, 50, 100, 200, 500, 1000, 2000, 5000和10000 每个培养孔,测量8个重复,细胞数量与荧光强度呈良好的线性关系,线性相关系数为0.9996746375

图2细胞TC-71(尤因氏肉瘤)3天,20000细胞

图3 96孔板测试系统荧光测量线性(r2 = 0.9996746375)
3、适用范围
(1)细胞毒性试验,通过DIMSCAN测量集落形成可以实现动态范围在4至7天内的生长曲线。


图4细胞毒性试验
Cell line TC-71 (Ewing's sarcoma), day 3, 20000 cells
Treated with 4-HPR + n-oleylethanolamine (nOE), 1:1 (0-12 um both)
(2)荧光扫描图象处理

图5 图象阈值处理(左边:原始图像;右边:处理后的二值图像)
(3)建立细胞荧光预览缩略图

图6 培养板单孔细胞荧光预览缩略图
(4)其他用途
免疫测定(细菌、病毒、毒素、病原体)和蛋白质组检测;核酸,DNA和基因组检测;快速即时的临床诊断;食品和环境检测;毒素检测
4、参数
倒置荧光显微镜:Microscope: Olympus IX50 inverted microscope
步进电动平移台:Prior Pro Scan, 步进电机200步/转; 250微步/整步; S曲线加速
(stepper motors 200 steps/revolution; 250 microsteps/full step; S-curve acceleration)
CCD相机: QImaging Retiga Exi
CCD分辨率:1024/768
拥有内部冷却功能
像素混合模式:1-4
(CCD resolution 1024/768;internal cooling;binning modes available: 1-4)
配置英特尔酷睿2双核处理器以上的计算机
基本配置:
奔腾III 800 MHz 或者更高;256 MB RAM或更高;
集成了IEEE1394火线接口的PCI卡
(Pentium III 800 MHz, 256 MB RAM; IEEE1394 FireWire interface PCI card)
操作系统(Operating System: Windows 2000,XP

图7 细胞培养板扫描过程图示
5、使用方法
(1)DIMSCAN用户界面组成
程序窗口的主要部分包括:
控制面板
——完整的板图片
——支持通过选定孔导航阶段
——任意孔扫描选择
——扫描完成该孔含有重建图像
——严重扫描孔重新扫描已启用
现场摄像头控制
——实时图像流
——相机控制,包括阈值
缩略图
——个别培养孔扫描高分辨率图像重建

图8 DIMSCAN 半自动荧光数字图像显微处理系统观察界面
(2)主要操作流程
根据具体说明书进行。
6、系统组成部分
倒置荧光显微镜,步进电机扫描器,扫描控制器,CCD像机(软件Qimaging Microimager Ⅱ),微型计算机。

图9 DIMSCAN观测系统的组成模块
参考文献
-
Proffitt RT, Tran JV, Reynolds CP. A fluorescence digital image microscopy system for quantitating relative cell numbers in tissue culture plates. Cytometry 24:204-213. 1996
-
Krejsa J, Frgala F, Alfaro P, Reynolds CP. DIMSCAN 3.0, A New Generation of Fluorescence Digital Image Microscopy System for Measuring Cytotoxicity in Microplates. 2002 AACR Annual Meeting (poster). 2002
-
Ondrej Kalous, Julie Watanabe, Kun Jung Lee, A. Linn Murphree, C. Patrick Reynolds. Use Of A Novel Microplate Fluorescence Cytotoxicity Assay (Dimscan-384) To Evaluate Combinations Of Cytotoxic Agents In A Panel Of Retinoblastoma Cell Lines. 2003 AACR Annual Meeting. 2003
-
Ondrej Kalous, Jiri Krejsa, Tomas Frgala, C. Patrick Reynolds. The DIMSCAN cytotoxicity assay, unlike the MTT assay, identifies syngergistic combinations of anticancer agents.. 2004 AACR Annual Meeting. 2004
-
Keshelava, N., Frgala, T., Krejsa, J., Kalous, O., and Reynolds, C. P.. DIMSCAN: a microcomputer fluorescence-based cytotoxicity assay for preclinical testing of combination chemotherapy.. Methods Mol.Med., 110: 139-153. 2005
-
Frgala T, Kalous O, Proffitt RT, Reynolds CP. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations.. Mol Cancer Ther, 6(3):886-97. 2007
ABSTRCT FOR DIMSCAN
DIMSCAN is a semi-automatic digital image microscopy system for quantitating relative cell numbers in tissue culture plates. Cytotoxicity assays measured by DIMSCAN using fluorescein diacetate (FDA), a dye accumulating selectively in viable cells, can achieve a 4 log dynamic range at 4 to 7 days and correlate with colony forming assays. The system consists of an inverted fluorescence microscope, stepper motor scanning stage, the stage controller, CCD camera and a microcomputer running the main application, which controls stage movement and processes CCD camera images. Relative numbers of viable cells are determined by evaluating FDA fluorescence intensity, with background fluorescence eliminated by digital thresholding and a quenching of florescence in non-viable cells with the vital stain Eosin Y. The current system uses Olympus IX50 inverted florescence microscope, a Prior motorized stage, a Qimaging Microimager II camera, and a Pentium III computer running Windows 2000. Rectangular plates can be employed with number of wells ranging from 6 to 96 wells per plate. Average scan time for a 96 well plate is 6 minutes.
|
apers on DIMSCAN development
|
Authors
|
Paper name
|
Published in
|
Year
|
|
Proffitt RT, Tran JV, Reynolds CP
|
A fluorescence digital image microscopy system for quantitating relative cell numbers in tissue culture plates
|
Cytometry 24:204-213
|
1996
|
|
Krejsa J, Frgala F, Alfaro P, Reynolds CP
|
DIMSCAN 3.0, A New Generation of Fluorescence Digital Image Microscopy System for Measuring Cytotoxicity in Microplates
|
2002 AACR Annual Meeting (poster)
|
2002
|
|
Ondrej Kalous, Julie Watanabe, Kun Jung Lee, A. Linn Murphree, C. Patrick Reynolds
|
Use Of A Novel Microplate Fluorescence Cytotoxicity Assay (Dimscan-384) To Evaluate Combinations Of Cytotoxic Agents In A Panel Of Retinoblastoma Cell Lines
|
2003 AACR Annual Meeting
|
2003
|
|
Ondrej Kalous, Jiri Krejsa, Tomas Frgala, C. Patrick Reynolds
|
The DIMSCAN cytotoxicity assay, unlike the MTT assay, identifies syngergistic combinations of anticancer agents.
|
2004 AACR Annual Meeting
|
2004
|
|
Keshelava, N., Frgala, T., Krejsa, J., Kalous, O., and Reynolds, C. P.
|
DIMSCAN: a microcomputer fluorescence-based cytotoxicity assay for preclinical testing of combination chemotherapy.
|
Methods Mol.Med., 110: 139-153
|
2005
|
|
Frgala T, Kalous O, Proffitt RT, Reynolds CP
|
A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations.
|
Mol Cancer Ther, 6(3):886-97
|
2007
|
|
[ Back to Top ]
Papers using DIMSCAN:
|
Authors
|
Paper name
|
Published in
|
Year
|
|
Reynolds CP, Schindler P, Jones D, Gentile J, Proffitt R, Einhorn P
|
Comparison of 13-cis- retinoic acid to trans-retinoic acid using human neuroblastoma cell lines
|
Advances in Neuroblastoma Research 4, Evans A, Biedler JL, Brodeur G, D'Angio GJ, Nakagawara A (eds.), New York: John Wiley & Sons, 237-244
|
1994
|
|
Keshelava N, Seeger RC, Reynolds CP
|
Drug resistance phenotype of neuroblastoma cell lines established at diagnosis and at relapse after induction chemotherapy or bone marrow transplantation
|
European J Cancer33:2002-2006
|
1997
|
|
Anderson CP, Tsai JM, Chan WW, Liu RM, Forman HJ, Reynolds CP
|
Buthionine sulfoximine (BSO) is cytotoxic via apoptosis and synergistically enhances the activity of melphalan (L-PAM) in human neuroblastoma cell lines
|
European J Cancer 33:2016-2019
|
1997
|
|
Keshelava N, Seeger RC, Groshen S, Reynolds CP
|
Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy
|
Cancer Research 58:5396-5405
|
1998
|
|
Anderson CP, Tsai JM, Meek WE, Liu RM, Tang Y, Forman HJ, Reynolds G P
|
Depletion of Glutathione (GSH) by buthionine sulfoximine (BSO) is cytotoxic for human neuroblastoma cell lines via apoptosis
|
Experimental Cell Research 246:183-192
|
1999
|
|
Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP
|
N-(4-hydroxypheynl)retinamide increases ceramide and reactive oxygen species and induces mixed apoptosis/necrosis in neuroblastoma cell lines
|
J Natl Cancer Inst 91:1138-1146
|
1999
|
|
Keshelava N, Groshen S, Reynolds CP
|
Cross-resistance of topoisomerase I and II inhibitors in neuroblastoma cell lines
|
Cancer Chemotherapy and Pharmacology45:1-8
|
2000
|
|
Chen RL, Reynolds CP, Seeger RC, Cabot MC, Reynolds CP
|
Neutrophils are cytotoxic and growth inhibiting for neuroblastoma cells with anti-GD2 antibody but without cytotoxicity can be growth stimulating
|
Clinical Immunol Immunother 48:603-612
|
2000
|
|
Maurer BJ, Cabot MC, Reynolds CP
|
Synergism of N-(4-hydroxyphenyl)retinamide cytotoxicity by modulators of ceramide metabolism in solid tumor cell lines
|
J Natl Cancer Inst 92:1897-1908
|
2000
|
|
Reynolds CP, Wang Y, Melton LJ, Einhorn PA, Slamon DJ, Maurer BJ
|
Retinoic-acid resistant neuroblastoma cell lines show altered myc regulation and high sensitivity to fenretinide
|
Medical Pediatric Oncology 35:597-602
|
2000
|
|
Keshelava N, Zuo JJ, Chen P, Waidyaratine SN, Luna MC, Gomer CJ, Triche CJ, Reynolds CP
|
Loss of p53 function confers high-level multi-drug resistance in neuroblastoma cell lines
|
Cancer Research 61:6185-6193
|
2001
|
|
Anderson CP, Seeger RC, Satake N, Meek WE, Keshelava N, Bailey HH, Monforte-Munoz HL, Reynolds CP
|
Buthionine sulfoximine and myeloablative concentrations of melphalan overcome resistance in a melphalan-resistant neuroblastoma cell line
|
J Pediat Hematol Oncol 23:500-505
|
2001
|
|
Metelitsa LS, Gillies SD, Super M, Shimada H, Reynolds CP, Seeger RC
|
Enhanced expression and activation of Mac-1(CD11b/CD18) by an anti-GD2/GM-CSF fusion protein increases neutrophil antibody dependent cellular cytotoxicity
|
Blood 99:4166-4173
|
2002
|
|
O'Donnell PH, Guo WX, Reynolds CP, Maurer BJ
|
N-(4-hydroxyphenyl)retinamide increases ceramide and is cytotoxic to acute lymphoblastic leukemia cell lines, but not to non-malignant lymphocytes
|
Leukemia 16:902-910
|
2002
|
|
Anderson C, Reynolds CP
|
Cytotoxicity of buthionine sulfoximine (BSO) and melphalan/BSO in combination for neuroblastoma cell lines derived after myeloablative therapy
|
Bone Marrow Transplantation 30:135-140
|
2002
|
|
Peter J. Houghton, Peter C. Adamson, Susan Blaney, Howard A. Fine, Richard Gorlick, Michelle Haber, Lee Helman, Steve Hirschfeld, Melinda G. Hollingshead, Mark A. Israel, Richard B. Lock, John M. Maris, Glenn Merlino, Wendy Patterson, C. Patrick Reynolds, Kevin Shannon, Alice Yu, John Yu, and Malcolm A. Smith
|
Testing of New Agents in Childhood Cancer Preclinical Models : Meeting Summary
|
Clinical Cancer Research 8:3646-3657
|
2002
|
|
Bo Yang, Nino Keshelava, Clarke P. Anderson, and C. Patrick Reynolds
|
Antagonism of Buthionine Sulfoximine Cytotoxicity for Human Neuroblastoma Cell Lines by Hypoxia Is Reversed by the Bioreductive Agent Tirapazamine
|
Cancer Research 63:1520-1526
|
2003
|
|
Nino Keshelava, Denice Tsao-Wei, and C. Patrick Reynolds
|
Pyrazoloacridine Is Active in Multidrug-resistant Neuroblastoma Cell Lines with Nonfunctional p531
|
Clinical Cancer Research 9:3492-3502
|
2003
|
|
Grigoryan, R., Keshelava, N., Anderson, C., and Reynolds, C. P.
|
In vitro testing of chemosensitivity in physiological hypoxia.
|
Methods Mol.Med., 110: 87-100
|
2005
|
|
Reynolds, C. P. and Maurer, B. J.
|
Evaluating response to antineoplastic drug combinations in tissue culture models.
|
Methods Mol.Med., 110: 173-183
|
2005
|
|
Yang, B. and Reynolds, C. P.
|
Tirapazamine cytotoxicity for neuroblastoma is p53 dependent.
|
Clin.Cancer Res., 11: 2774-2780
|
2005
|
|
Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Tony Ng T, Reynolds CP, Triche TJ, Sorensen PHB
|
E-cadherin cell-cell adhesion in Ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase.
|
Cancer Research, 67: 3094-3105
|
2007
|
|
Houghton PJ, Morton CL, Tucker C, Gorlick R, Kolb EA, Zhang W, Lock R, Carol H, Reynolds CP, Keshelava N, Maris JM, Courtright J, Keir ST, Friedman HS, Stopford C, Wu J, Smith MA
|
Stage 1 testing of the proteasome inhibitor bortezomib by the Pediatric Preclinical Testing Program.
|
Pediatric Blood & Cancer, (In Press).
|
2007
|
|
Keshelava N, Davicioni E, Zesheng Wan Z, Ji L, Sposto R, Triche TJ, Reynolds CP
|
Inhibition of histone deacetylase (HDAC) 1, a drug target identified by expression profiling, sensitizes multi-drug-resistant neuroblastoma cell lines to cytotoxic agents.
|
J National Cancer Institute, 99:1107-19
|
2007
|
|
Maris JM, Courtright J, Houghton PJ, Morton CL, Gorlick R, Kolb EA, Lock R, Tajbakhsh M, Reynolds CP, Keir ST, Wu J, Smith MA
|
Initial testing (Stage 1) of the VEGFR inhibitor AZD2171 by the Pediatric Preclinical Testing Program.
|
Pediatric Blood & Cancer., (In Press)
|
2007
|
|
Kang MH, Kang YH, Szymanska B, Wilczynska-Kalak U, Sheard MA, Harned T, Lock RB, Reynolds CP
|
Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo.
|
Blood, 110:205-2066
|
2007
|
|
Tajbakhsh M, Houghton PJ, Morton CL, Kolb EA, Maris JM, Keir ST, Wu J, Reynolds CP, Smith MA, Lock RB
|
Initial testing (Stage 1) of cisplatin by the Pediatric Preclinical Testing Program.
|
Pediatric Blood & Cancer, (In Press)
|
2007
|
|
Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock R, Tajbakhsh M, Reynolds CP, Maris JM, Keir ST, Billups CA, Smith MA
|
Initial testing of dasatinib by the Pediatric Preclinical Testing Program.
|
Pediatric Blood & Cancer, (In Press)
|
2007
|
|
Reynolds CP, Kang MH, Keshelava N, Mauer BJ
|
Assessing combinations of cytotoxic agents using leukemia cell lines.
|
Current Drug Targets, 8:765-771
|
2007
|
|
[ Back to Top ]
Clinical Trials Enabled by DIMSCAN Testing:
|
Authors
|
Paper name
|
Published in
|
Year
|
|
Villablanca JG, Avramis V, Khan A, Matthay KK, Ram say NKC, Seeger RC, Reynolds CP
|
Phase I trial of 13-cis-retinoic acid (cRA) in neuroblastoma patients following bone marrow transplantation (BMT)
|
J Clinical Oncology13:894-901
|
1995
|
|
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shamada H, Black CG, Brodeur GM, Gerbing R, Reynolds CP
|
Treatment of high risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid
|
New Eng J Med 341:1165-1173
|
1999
|
|
Villablanca JG, Ames MM, Reid JM, Bagniewski P, Krail M, Reynolds CP
|
Phase I trial of oral [N- (-4-hydroxyphenyl) retinamide] (4-HPR) in children with resistant/recurrent solid tumors: A Children's Cancer Group Study (CCG 09709)
|
Proc Amer Soc Clin Oncol21:398a
J Clinical Oncology24:3423-3430
|
2002
2006
|
|
Anderson CP, Robert Seeger RC, Bailey H, Reynolds CP
|
Pilot of buthionine sulfoximine (BSO) combined with non-myeloablative melphalan (L-PAM) against refractory neuroblastoma (NB)
|
Proc Amer Soc Clin Oncol21 :298a
|
2002
|
|
New Approaches to Neuroblastoma Therapy
|
N2002-01: A Phase I Study of High-Dose Pyrazoloacridine (PZA) (NSC 366140) Supported with Autologous Hematopoietic Stem Cell Rescue in Children with Recurrent or Resistant Neuroblastoma.
|
Closed
|
|
|
Children's Oncology Group
|
ANBL0321: A Phase II Study of Fenretinide in Children with Recurrent/Resistant High Risk Neuroblastoma.
|
Ongoing
|
|
|
New Approaches to Neuroblastoma Therapy
|
N99-02: A Phase I trial of BSO + L-PAM and Stem Cell Support.
|
Ongoing
|
|
|
Norris Cancer Center
|
A Phase II trial of Fenretinide in Recurrent Ovarian Cancer.
|
Proc Amer Soc Clin Oncol23:461
Proc Amer Soc Clin Oncol25: Abst 5555
|
2004
2007
|
|
California Cancer Consortium
|
A Phase II trial of Fenretinide in Prostate Cancer.
|
Ongoing
|
|
|
California Cancer Consortium
|
A Phase I trial of Intravenous Fenretinide in Hematological Malignancies.
|
Ongoing
|
|
|
California Cancer Consortium
|
A phase I trial of intravenous fenretinide in solid tumors
|
Ongoing
|
|
|
California Cancer Consortium
|
A phase II trial of fenretinide in asymptomatic rising PSA prostate cancer
|
Completed
|
|
|
New Approaches to Neuroblastoma Therapy
|
N2004-04: A Phase I Study of Fenretinide Lym-X-SorbTM (LXS) Oral Powder in Patients with Recurrent or Resistant Neuroblastoma (IND # 68,254)
|
Ongoing
|
|
|
Therapeutic Advances in Childhood Leukemia
|
TACL 2005-001: A phase I/II trial of ABT-751 combined with dexamethasone, PEG-asparaginase, and doxorubicin in relapsed acute lymphoblastic leukemia (ALL)
|
Ongoing
|
|
|
Therapeutic Advances in Childhood Leukemia
|
TACL 2005-003: Bortezomib with chemotherapy for relapsed childhood ALL.
|
Ongoing
|
|
|
Therapeutic Advances in Childhood Leukemia
|
TACL 2006-001: IV Fenretinide in Relapsed ALL, AML or NHL.
|
Ongoing
|
|
|
|