| 产品名称:高通量细胞间相互作用自动分析系统,单细胞动态分析系统-单个细胞水平高通量自动分析细胞与细胞之间相互作用 |
| 品牌:欧美进口 |
| 货号: |
| 价格:询价 |
| 联系人:李先生 |
| 电话:18618101725 |
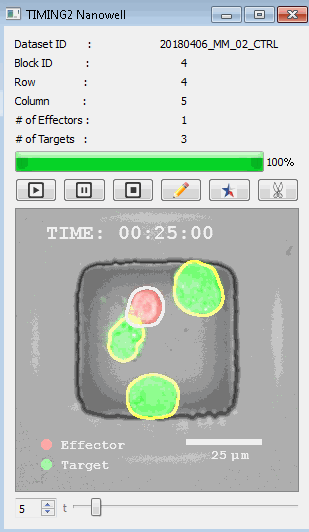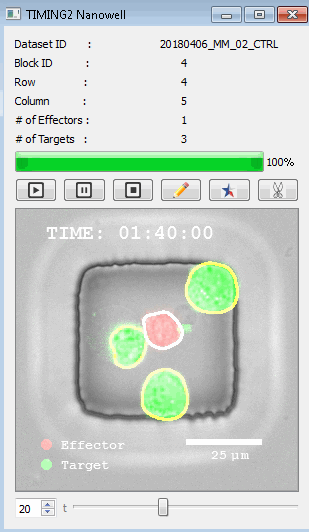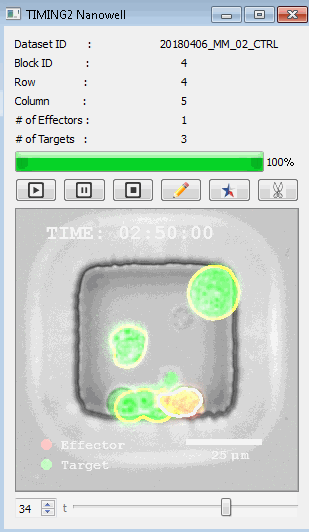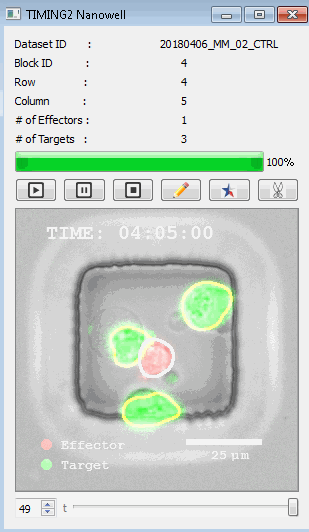
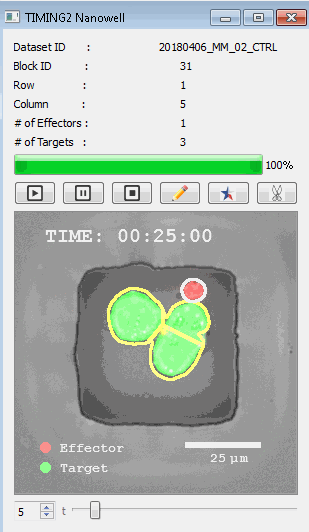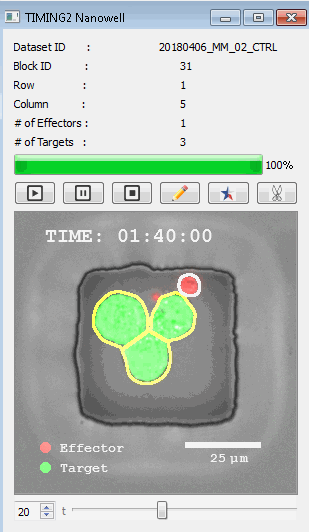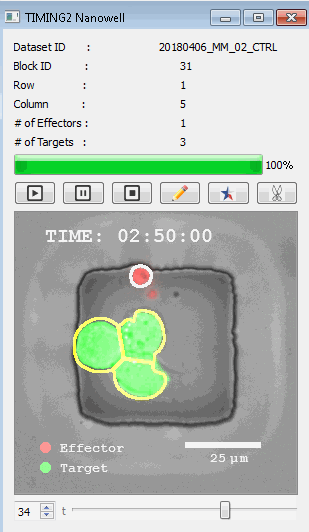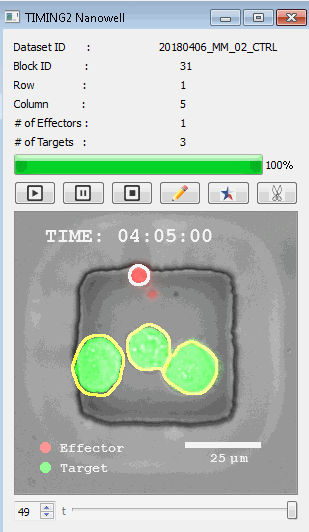
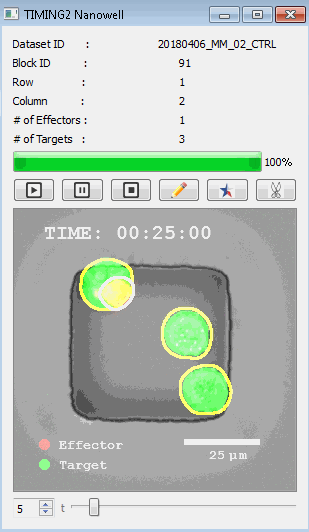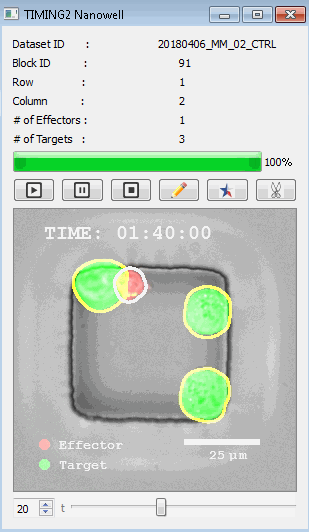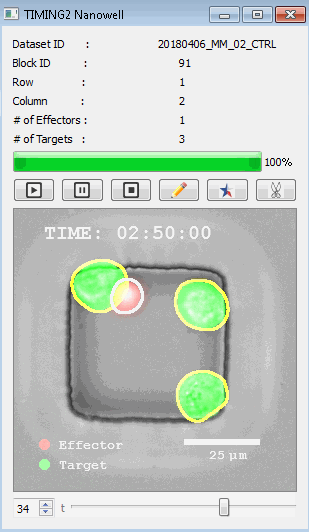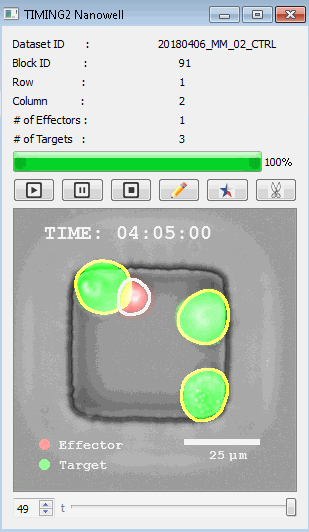
高通量单细胞水平细胞间相互作用自动分析系统 该平台提供了对单个细胞如何移动、行为、交互和表现的高通量评估
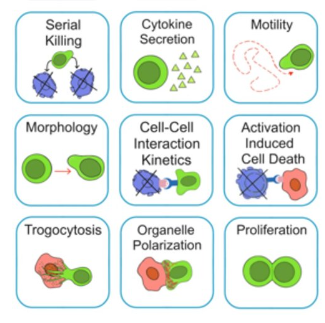
了解哪些细胞表现佳以及原因 评估细胞激活、杀伤和运动间的变化 一可同时评估单个细胞如何移动、激活、相互作用、杀死和生存的单细胞技术 科学家依靠该系统平台量化数千个免疫细胞的时空性能,以了解哪些候选细胞进入临床试验、了解临床反应并保持生产的一致性
平台广泛适用于各种细胞类型和应用。虽然生命科学研究人员受益于单细胞分析的重大,但该系统超越了静态分析。通过该系统,生命科学创新者可以观察新的生物学,并对细胞如何移动、激活、杀死和生存获得新的、意想不到的理解。 纳米阱网格是一种广泛适用的、实用的方法,用于记录体外动态细胞-细胞相互作用,提供了广泛统计采样所需的大量吞吐量。重要的是,当图像分析揭示出特别感兴趣的细胞时,它们的坐标是足够的,可以使机器人检索用于克隆扩增或PCR等下游处理。在癌症免疫治疗的背景下,该方法可以监测效应器介导的对所需靶细胞的细胞毒性,而无需靶细胞工程。这提供了重要的优势,例如使用自体或匹配/原代肿瘤细胞作为靶细胞的能力。 该系统从纳米阱网格中细胞的高通量延时成像显微镜数据中自动分析细胞-细胞相互作用,为免疫疗法中的细胞-细胞交互作用提供了基本见解。 该系统广泛适用于一般免疫学、细胞生物制造、癌症生物学、分化、干细胞工程、基于血液中不同细胞类型之间相互作用的药物筛选中动态细胞-细胞相互作用的高通量定量研究。 该系统执行大规模自动化视频阵列分析。它提供了一个快速的“交钥匙”解决方案,具有高效的用户界面,以单细胞分辨率生成细胞相互作用行为的定量分析, 具有高产量、自动化、速度和准确性,并具有高效的视觉确认。 它使用了利用纳米阱空间限制的分割和跟踪算法,实现了98%以上的细胞检测、分割和跟踪精度,而这些精度是无法直接实现的。该系统在集中式服务器上运行, 需要在服务器之间进行耗时的数据传输。 它自动计数每个纳米井中的细胞,根据细胞类型标记识别细胞类型,并提供细胞大小、位置、形状和运动的动态测量;细胞-细胞接触的频率和持续时间, 以及细胞事件荧光标记物的变化。它实现了这些测量的链接可视化和分析,并具有快速编辑功能。为了实现下游统计分析和假设测试,我们软件中的数据可以以电子表格的形式导出
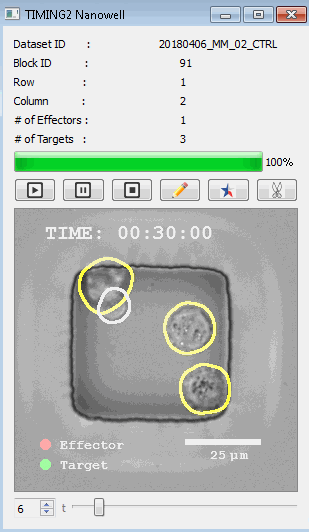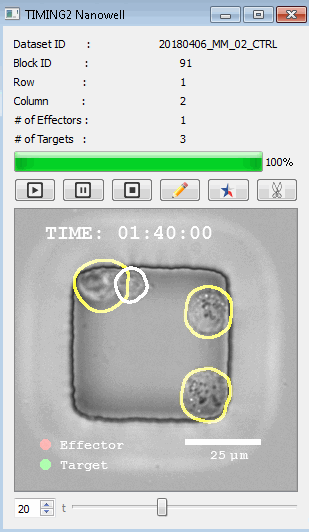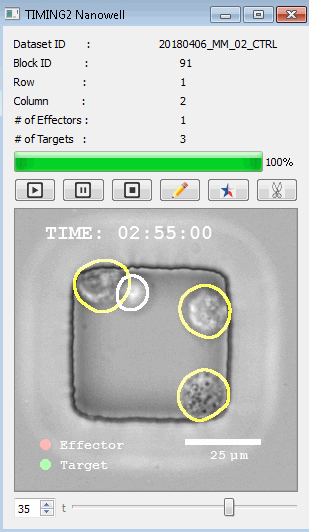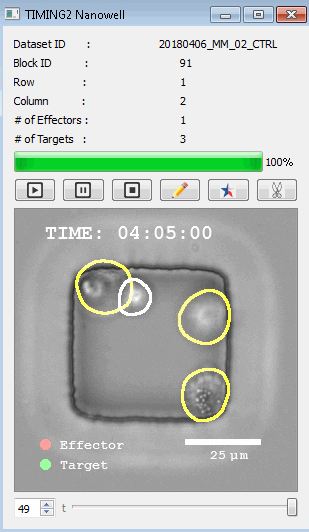
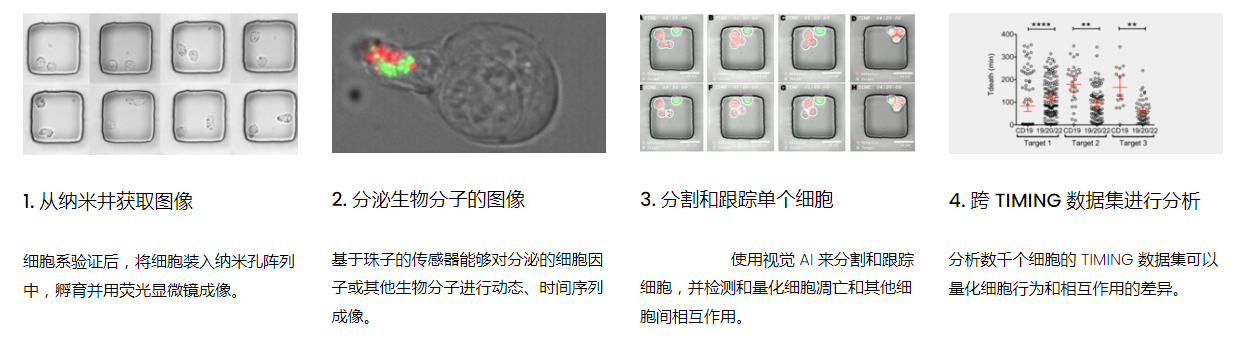
工作流程: 从客户处收到冷冻保存的效应细胞和靶细胞后,该系统解冻并进行细胞系活力研究。还可以运行客户特定的检测,以确保细胞以预期的方式表现。此时,执行其单细胞工作流程如下:
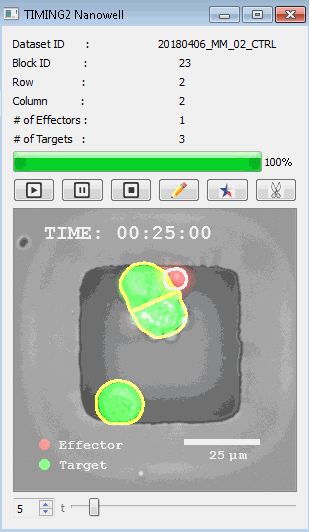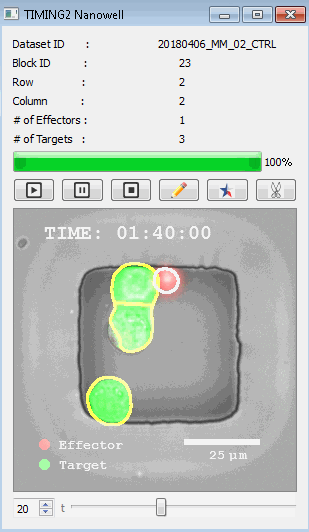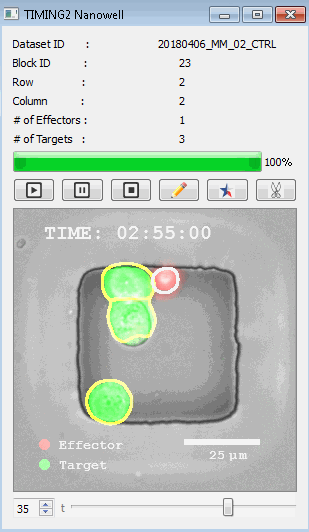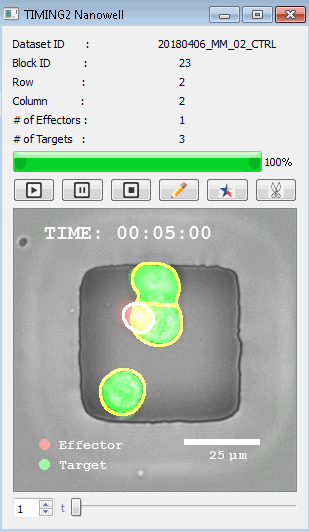
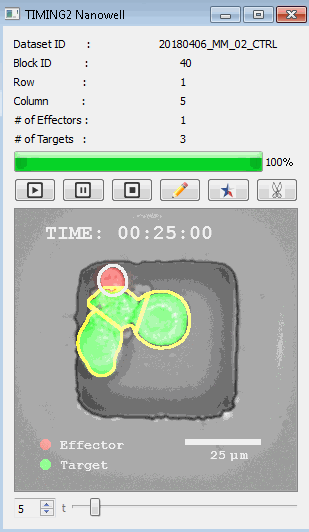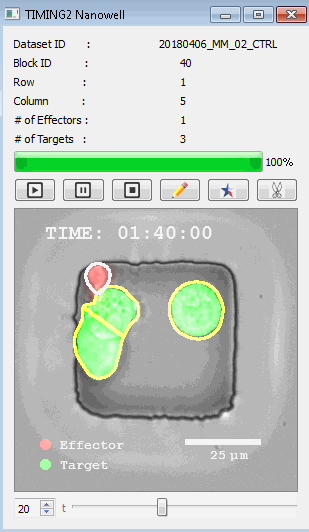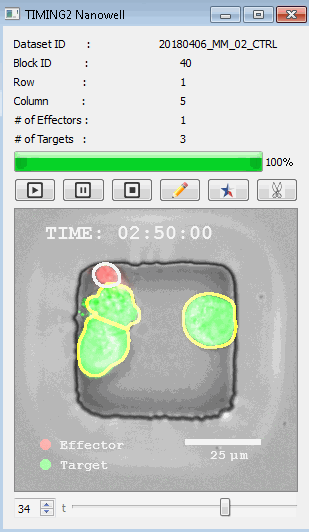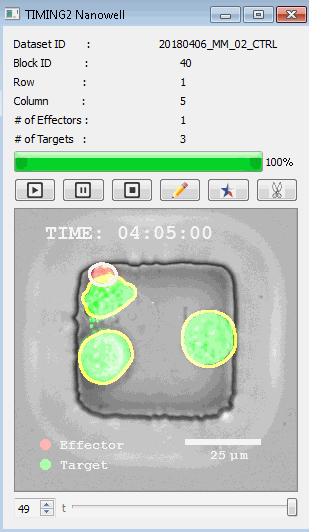
DISCover 工作流程 TIMING分析使该系统能够识别感兴趣的细胞以进行进一步分析,从而更面地了解细胞的行为方式。 直观地评估细胞间的表现和相互作用的情况 纳米孔网格延时成像显微镜 (TIMING) 应用基于神经网络的检测来评估数千个单个细胞与细胞的相互作用 表征数千个细胞的迁移、接触动力学和细胞死亡 该系统应用人工智能并行进行数千个显微镜实验,以高通量和单细胞分辨率表征迁移、细胞间相互作用、细胞毒性、存活和生物分子分泌
将运动和性能与多组学分析相结合 可以识别和检索感兴趣的细胞,将功能分析与单细胞 RNA 测序和流式细胞术等分析方式的信息联系起来,从而提供对细胞功能、状态和表型的面了解。
PublicationsThe CellChorus platform is the only approach that can associate motility, contact, killing, cytokine secretion and other dynamic readouts over time for individual cells and cell-cell interactions at high throughput. This approach is called dynamic single-cell analysis. The platform has broad application across antibodies, cell therapies, vaccines and other technologies, as shown in the publications below from leading journals such as Blood, Clinical Cancer Research, Nature Immunology, the JITC, Journal of Immunology, and Science Advances. In less than 18 months, the FuseBio team developed a TCE lead candidate that targets ROR1 (FUSE394). The data presented at SITC 2022 demonstrated that FUSE394 is potent and successfully decouples anti-tumor activity from cytokine release and T cell exhaustion. TIMING data from the CellChorus early access program demonstrated that FUSE394 preserves T cell motility and healthy morphology and promotes 50% more synapse formation than a non-decoupled control. Researchers applied the TIMING platform to test NK-mediated cytotoxicity at single-cell resolution as part of research showing that chimeric antigen receptor (CAR) activation in natural killer (NK) cells promoted transfer of the CAR cognate antigen from tumor to NK cells, resulting in (1) lower tumor antigen density, thus impairing the ability of CAR-NK cells to engage with their target, and (2) induced self-recognon and continuous CAR-mediated engagement, resulting in fratricide of trogocytic antigen-expressing NK cells (NKTROG+) and NK cell hyporesponsiveness. Researchers applied the CellChorus TIMING platform to evaluate interactions between thousands of tumor cells and CAR-T cells that were to be infused into patients to preview how the CAR-T cells being given to patients can eradicate tumor cells after infusion. The researchers robotically selected super-killer T cells for transcriptional profiling to characterize the molecular properties of the CAR-T cells with the best features for eradicating tumors. Time-lapse imaging microscopy in nanowell grids (TIMING?) profiling revealed that T cells from responders showed migration (persistent motion for at least one body length), and migration was associated with serial killing capacity. In addon, confocal microscopy revealed that migration is linearly correlated with both mitochondrial volume and lysosomal volume; and scRNA-seq demonstrated that T cells from responders were enriched in pathways related to T-cell killing, migration and actin cytoskeleton, and TCR clustering. By simultaneously evaluating thousands of individual interactions between T cells and target cells bearing virally derived peptides, the company’s artificial intelligence-powered TIMING? platform reveals individual T cells that are capable of polyfunctionality based on killing, serial killing, and secretion of the cytokine interferon gamma (IFNγ). Time-lapse imaging in nanoliter wells was applied to understand the kinetics of translocation at the single‐cell level and to enable tracking of the same individual cells. The micromesh array contains nanoliter wells that enabled imaging protein translocation dynamically. Understanding why only some T cells are capable of killing, and identifying mechanisms that can improve killing has remained elusive. These results illustrate that while non-killer T cells are reflective of population heterogeneity, integrated single-cell profiling can enable identification of mechanisms that can enhance the function/proliferation of killer T cells leading to direct anti-tumor benefit. Using time-lapse imaging microscopy to monitor T cell-mediated tumor killing at the single cell level to gain more complete understanding of the kinetics of killing in studies that reveal two distinct types of immune resistance regulators and demonstrate their potential as therapeutic targets to improve the efficacy of immunotherapy. Immune cells such as T cells and natural killer cells, and target cells such as NALM6, K562 and EL4, were incubated in PDMS nanowell arrays and imaged using time-lapse fluorescent microscopy. The proposed cell segmentation and tracking algorithms allowed automated quantification of cell pairs, cell location, morphology, interactions and movement as well as cell viability, without the need for manual processing. The result revealed that cytotoxic T cells have higher motility than noncytotoxic T cells, both before and during synapse formation. We found sustained activation of cytotoxicity, costimulation, oxidative phosphorylation– and proliferation-related genes, and simultaneously reduced differentiation and exhaustion. Our study identifies molecular features of TCR8 expression that can guide the development of enhanced immunotherapies. CD19/20/22CAR T-cells killed CD19(?) blasts from patients who relapsed after CD19CAR T-cell therapy and CRISPR/Cas9 CD19 knockout primary BL-ALL both in vitro and in an animal model, while CD19CAR T-cells were ineffective. At the subcellular level, CD19/20/22CAR T-cells formed dense immune synapses with target cells that mediated effective cytolytic complex formation, were efficient serial killers in single-cell tracking studies, and were as efficacious as CD19CAR T-cells against primary CD19(+) disease. In conclusion, independent of CD19 expression, CD19/20/22CAR T-cells could be used as salvage or front-line CAR therapy for patients with recalcitrant disease. By utilizing both tradonal blob detection to generate binary mask labels from the stained channel images and the deep learning Mask RCNN model to train a detection and segmentation model, we managed to segment nuclei based only on phase images. The detection average precision is 0.82 when the IoU threshold is to be set 0.5. And the mean IoU for masks generated from phase images and ground truth masks from experts is 0.735. Without any ground truth mask labels during the training time, this is good enough to prove our hypothesis. This result enables the ability to detect nuclei without the need for exogenous labeling. This paper proposes an efficient variant of capsule networks (CapsNets) as an alternative to CNNs. Extensive experimental results demonstrate that the proposed CapsNets achieve competve performances in target cell apoptosis classification, while significantly outperforming CNNs when the number of training samples is small. To utilize temporal information within microscopy videos, we propose a recurrent CapsNet constructed by stacking a CapsNet and a bi-directional long short-term recurrent structure. Our experiments show that when considering temporal constraints, the recurrent CapsNet achieves 93.8% accuracy and makes significantly more consistent prediction than NNs. Integration of transcriptomic profiling, immune phenotyping and metabolism demonstrated that motile cells are more na?ve-like with higher oxidative metabolism and spare respiratory capacity. Our result also revealed that the master metabolic regulator AMP kinase (AMPK) is required for CAR+ T cells with high motility. We used a xenograft leukemia mouse model (CD19+ NALM-6) and validated that the motile cells have enhanced persistence and superior anti-cancer effect in vivo compared to the parental un-sorted population. Collectively, our multi-dimensional results demonstrated that persistent motility is a selectable biomarker of expanded CAR+ T cell bioactivity. On a desktop computer, TIMING 2.0 takes 5 s/block/image frame, four times faster than our previous method on the same computer, and twice as fast as our previous method (TIMING) running on a Dell PowerEdge server. The cell segmentation accuracy (f-number?=?0.993) is superior to our previous method (f-number?=?0.821). A graphical user interface provides the ability to inspect the video analysis results, make corrective edits efficiently (one-click edng of an entire nanowell video sequence in 5–10 s) and display a summary of the cell killing efficacy measurements. Genetically engineered T cells that express chimeric antigen receptors (CAR+) are heterogeneous and thus, understanding the immunotherapeutic efficacy remains a challenge in adoptive cell therapy. We developed a high-throughput single-cell methodology, Timelapse Imaging Microscopy In Nanowell Grids (TIMING) to monitor interactions between immune cells and tumor cells in vitro. Using TIMING we demonstrated that CD4+ CAR+ T cells participate in multi-killing and benefit from improved resistance to activation induced cell death in comparison to CD8+ CAR+ T cells. For both subsets of cells, effector cell fate at the single-cell level was dependent on functional activation through multiple tumor cells. A comprehensive understanding of the polyfunctionality of T lymphocytes in ICI or adoptive cell transfer (ACT), at single-cell resolution, will quantify T-cell properties that are essential for therapeutic benefit. We briefly highlight several emerging integrated single-cell technologies focusing on the profiling of multiple properties/functionales of T cells. We envision that these tools have the potential to provide valuable experimental and clinical insights on T-cell biology, and eventually pave the road for the discovery of surrogate T-cell biomarkers for immunotherapy. CD8+BTLA- TILs could not control tumor growth in vivo as well as their BTLA+ counterpart and antigen-specific CD8+BTLA- T cells had impaired recall response to a vaccine. However, CD8+BTLA+ TILs displayed improved survival following the killing of a tumor target and heightened "serial killing" capacity. Using mutants of BTLA signaling motifs, we uncovered a costimulatory function mediated by Grb2 through enhancing the secretion of IL-2 and the activation of Src after TCR stimulation. Our data portrays BTLA as a molecule with the singular ability to provide both costimulatory and coinhibitory signals to activated CD8+ T cells, resulting in extended survival, improved tumor control, and the development of a functional recall response. Clin Cancer Res; 23(20); 6151-64. ?2017 AACR. We used the engineered Fc domains to demonstrate in vitro and in mouse models that for therapeutic antibodies, complement-dependent cell-mediated cytotoxicity (CDCC) and complement-dependent cell-mediated phagocytosis (CDCP) by immunological effector molecules mediated the clearance of target cells with kinetics and efficacy comparable to those of the FcγR-dependent effector functions that are much better studied, while they circumvented certain adverse reactions associated with FcγR engagement. Collectively, our data highlight the importance of CDCC and CDCP in monoclonal-antibody function and provide an experimental approach for delineating the effect of complement-dependent effector-cell engagement in various therapeutic settings. Analysis of hundreds of individual human peripheral blood NK cells profiled ex vivo revealed that CD56dimCD16+ NK cells are immediate secretors of interferon gamma (IFN-γ) upon activation by phorbol 12-myristate 13-acetate (PMA) and ionomycin (< 3 h), and that there was no evidence of cooperation between NK cells leading to either synergistic activation or faster IFN-γ secretion. Furthermore, we observed that both the amount and rate of IFN-γ secretion from individual NK cells were donor-dependent. Collectively, these results establish our methodology as an investigational tool for combining phenotyping and real-time protein secretion of individual cells in a high-throughput manner. In aggregate, these results demonstrate the utility of our TIMING single cell methodology in uncovering not only the dynamic profile of T-cell behavior but also the ability to identify subpopulations of T-cell with enhanced polyfunctionality. Our studies support the use of motility as a surrogate and selective marker of higher CAR+ T cell bioactivity. These results also open up avenues to molecularly engineer T cells for an increased motility that could translate to better in vivo outcomes. Fluorescently labeled human T cells, natural killer cells (NK), and various target cells (NALM6, K562, EL4) were co-incubated on polydimethylsiloxane arrays of sub-nanoliter wells (nanowells), and imaged using multi-channel time-lapse microscopy. The proposed cell segmentation and tracking algorithms account for cell variability and exploit the nanowell confinement property to increase the yield of correctly analyzed nanowells from 45% (existing algorithms) to 98% for wells containing one effector and a single target, enabling automated quantification of cell locations, morphologies, movements, interactions, and deaths without the need for manual proofreading. Automated analysis of recordings from 12 different experiments demonstrated automated nanowell delineation accuracy >99%, automated cell segmentation accuracy >95%, and automated cell tracking accuracy of 90%, with default parameters, despite variations in illumination, staining, imaging noise, cell morphology, and cell clustering. An example analysis revealed that NK cells efficiently discriminate between live and dead targets by altering the duration of conjugation. The data also demonstrated that cytotoxic cells display higher motility than non-killers, both before and during contact. We implemented Timelapse Imaging Microscopy in Nanowell Grids (TIMING) to provide direct evidence that CD4(+)CAR(+) T cells (CAR4 cells) can engage in multikilling via simultaneous conjugation to multiple tumor cells. Comparisons of the CAR4 cells and CD8(+)CAR(+) T cells (CAR8 cells) demonstrate that, although CAR4 cells can participate in killing and multikilling, they do so at slower rates, likely due to the lower granzyme B content. Significantly, in both sets of T cells, a minor subpopulation of individual T cells identified by their high motility demonstrated efficient killing of single tumor cells. A comparison of the multikiller and single-killer CAR(+) T cells revealed that the propensity and kinetics of T-cell apoptosis were modulated by the number of functional conjugations. T cells underwent rapid apoptosis, and at higher frequencies, when conjugated to single tumor cells in isolation, and this effect was more pronounced on CAR8 cells. Our results suggest that the ability of CAR(+) T cells to participate in multikilling should be evaluated in the context of their ability to resist activation-induced cell death. We anticipate that TIMING may be used to rapidly determine the potency of T-cell populations and may facilitate the design and manufacture of next-generation CAR(+) T cells with improved efficacy. We demonstrate that the DLE-HuM195 antibody increases both the quality and the quantity of NK cell-mediated antibody-dependent cytotoxicity by endowing more NK cells to participate in cytotoxicity via accrued CD16-mediated signaling and by increasing serial killing of target cells. NK cells encountering targets coated with DLE-HuM195 induce rapid target cell apoptosis by promoting simultaneous conjugates to multiple target cells and induce apoptosis in twice the number of target cells within the same period as the wild-type mAb. Enhanced target killing was also associated with increased frequency of NK cells undergoing apoptosis, but this effect was donor-dependent. Antibody-based therapies targeting tumor antigens will benefit from a better understanding of cell-mediated tumor elimination, and our work opens further opportunes for the therapeutic targeting of CD33 in the treatment of acute myeloid leukemia. |